Difference between revisions of "Electroplating (or Galvanic Deposition)"
Doduco Admin (talk | contribs) (→Electroplating of Parts) |
Doduco Admin (talk | contribs) (→Electroplating of Parts) |
||
| Line 317: | Line 317: | ||
<figure id="fig:Electroplated Parts"> | <figure id="fig:Electroplated Parts"> | ||
| − | [[File:Electroplated Parts.jpg|left | + | [[File:Electroplated Parts.jpg|left|Electroplated Parts]] |
</figure> | </figure> | ||
Revision as of 08:08, 11 January 2023
For the electroplating of metals, especially precious metals and water based solutions (electrolytes) are used, which contain the metals to be deposited as ions (i.e. dissolved metal salts). An electric field between the anode and the work pieces as the cathode, forces the positively charged metal ions to move to the cathode where they give up their charge and deposit themselves as metal on the surface of the work piece. Depending on the application, for electric and electronic or decorative end use, different electrolytic bath solutions (electrolytes) are used. The electroplating equipment used for precious metal plating and its complexity, varies widely depending on the process technologies employed. Electroplating processes are encompassing besides the pure metal deposition also preparative and post treatments of the goods to be coated. An important parameter for creating strongly adhering deposits, is the surface of the goods to be metallic clean without oily or oxide film residues. This is achieved through various pre-treatment processes, specifically developed for the types of material and surface conditions of the goods to be plated. In the following segments, electrolytes – both precious and non-precious – as well as the most widely used electroplating processes are described.
Contents
Electroplating Solutions – Electrolytes
The actual metal deposition occurs in the electrolytic solution which contains the plating material as metal ions. Besides this basic ingredient, the electrolytes contain additional components depending on the processes used, such as for example conduction salts, brighteners and organic additives which are codeposited into the coatings, influencing the final properties of the electroplating deposit.
Precious Metal Electrolytes
All precious metals can be electroplated with silver and gold, also they are by far the most widely used ones (Table 1 and Table 2). The following precious metal electrolytes are the most important ones:
- Gold electrolytes
For functional and decorative purposes pure gold, hard gold, low-karat gold or colored gold coatings are deposited. Depending on the requirements, acidic, neutral or cyanide electrolytes based on potassium gold cyanide or cyanide free and neutral electrolytes based on gold sulfite complexes are used.
- Palladium and Platinum electrolytes
Palladium is mostly deposited as a pure metal, for applications in electrical contacts however also as palladium nickel. For higher value jewelry, allergy protective palladium intermediate layers are used as a diffusion barrier over copper alloy substrate materials. Platinum is mostly used as a surface layer on jewelry items.
- Ruthenium electrolytes
Ruthenium coatings are mostly used for decorative purposes, creating a fashionable “grey” ruthenium color on the surface. An additional color variation is created by using “ruthenium-black” deposits which are mainly used in bi-color decorative articles.
- Rhodium electrolytes
Rhodium deposits are extremely hard (HV 700 – 1000) and wear resistant. They also excel in light reflection. Both properties are of value for technical as well as decorative applications. While technical applications mainly require hard, stress and crack free coatings, the jewelry industry takes advantage of the light whitish deposits with high corrosion resistance.
- Silver electrolytes
Silver electrolytes without additives generate dull soft deposits (HV ~ 80) which are mainly used as contact layers on connectors with limited insertion and withdrawal cycles. Properties required for decorative purposes, such as shiny bright surfaces and higher wear resistance, are achieved through various additives to the basic Ag electrolyte.
| Type of Electrolyte | pH-Range | Deposit Properties Hardness | Areas of Application | |
|---|---|---|---|---|
| HV | Purity [kt] | |||
| Gold electrolytes | ||||
| AUROMET TN | 3.2 - 4.2 | ca. 70 | 99.99% Au | Base-deposits |
| AUROMET XPH | 0.3 - 0.6 | 160 - 180 | 99.8% Au | Base-deposits for stainless steel etc. |
| DODUREX COC | 4.6 - 4.9 | 160 - 180 | 99.6% Au | Printed circuit boards, connectors, contact parts, etc.; hard gold coatings for rack and barrel plating |
| DODUREX HS 100 | 4.3 - 4.6 | 160 - 180 | 99.6% Au | High speed process for connectors and PCB plating |
| PURAMET 202 PURAMET 402 |
5.5 - 6.5 7.0 - 7.5 |
60 - 80 60 - 80 |
99.99% Au 99.99% Au |
High purity gold coatings for electrical and electronic parts incl. semi conductors and PCBs; for demanding requirement on bonding properties |
| Platinum metal electrolytes | ||||
| RHODOPLAT T | strongly acidic | 900 | 99.0% Rh | Ductile rhodium deposits for thicker layers, reed contacts, sliding contacts |
| RUTHENIUMBAD | stronglyacidic | 900 | 99.0% Ru | Crack free thick ruthenium deposits |
| PLATINBAD 5 | stronglyacidic | 240 - 260 | 99.9% Pt | High temperature switching devices, etc |
| DODUPAL 3 | 7.0 - 8.0 | 220 - 250 | 99.9% Pd | Thin palladium layers as diffusion barrier |
| DODUPAL 5 | 7.0 - 8.0 | 220 - 250 | 99.9% Pd | Connectors and contact parts |
| DODUPAL 10 | 8.0 - 8.5 | 350 - 400 | 80.0% Pd | Pd/Ni for connectors and contact parts |
| Silver electrolytes | ||||
| ARGOL 30 | cyanidebased | a pprox. 90 | 99.9% Ag | Contact parts, connectors |
| ARGOL HS 100 | approx. 9.0 | 90 - 120 | 99.9% Ag | |
| ARGOL 2000 | approx. 12.0 | |||
| ARGOL 400 | 160 - 180 | |||
Non-Precious Metal Electrolytes
The most important non-precious metals that are deposited by electroplating are: Copper, nickel, tin and zinc as well as their alloys. The deposition is performed in the form of pure metals with different electrolytes used (Table 3).
- Copper electrolytes
Copper electrolytes are used for either depositing an intermediate layer on strips or parts, for building up a printed circuit board structure or for the final strengthening during the production of printed circuit boards.
- Tin electrolytes
Pure tin and tin alloy deposits are used as dull or also bright surface layers on surfaces required for soldering. In the printed circuit board manufacturing, they are also utilized as an etch resist for the conductive pattern design after initial copper electroplating.
| Type of Electrolyte | pH-Bereich | Deposit Properties | Areas of Application | |
|---|---|---|---|---|
| Hardness HV | Purity [kt] | |||
| Gold electrolytes | ||||
| DURAMET 1N14 DURAMET 2N18 DURAMET 3N DURAMET 265S DURAMET 333S DURAMET 386S |
3.4 - 3.8 3.4 - 3.8 3.4 - 3.8 3.4 - 3.8 3.2 - 3.6 3.4 - 3.8 |
1N 2N 3N Hamilton 1N Hamilton |
23 23 23 23 23 23 |
Jewelry, watches, writing instruments, frames for glasses, fixtures |
| HELODOR 630 | 8.5 - 9.5 | rose colored | 22 | Frames for glasses, jewelry, watches, writing instruments |
| DODUPLAT Y18 DODUPLAT Y18HS |
9.5 - 10.5 9.5 - 11 |
2N 2N |
18 16 |
Jewelry, watches, writing instruments |
| AUROMET TN AUROMET 2 AUROMET 4 |
3.2 - 4.2 3.2 - 4.0 3.2 - 4.2 |
Pure Gold 2 - 3N 2 - 3N |
23 23 23 |
Colored gold for jewelry, watches, writing instruments, fixtures, frames for glasses, etc. |
| Platinum metal electrolytes | ||||
| RHODIOR 2 RHODIOR 20 RHODIOR 25 RHODIOR 40 |
< 1 < 1 < 1 < 1 |
white white white white |
99.99%Rh 99.99%Rh 99.99%Rh 99.99%Rh |
Bright white rhodium coatings with high hardness for jewelry, watches, writing instruments, etc. |
| RUTHENIUMBAD | strongly acidic | grey/black | 99.0%Ru | Very hard and luster retaining Ruthenium coating |
| PLATINBAD | strongly acidic | white | 99.9%Pt | Jewelry, watches, etc. |
| DODUPAL 3 | 7.0 - 7.6 | Pd - color | 95%Pd | Thin Pd/Zn coating as Ni-free diffusion barrier |
| DODUPAL 10 | 8.0 - 8.5 | white | 80%Pd | Pd/Zn alloy for jewelry |
| DODUPAL 12 | 7.0 - 8.0 | white | 95%Pd | Pd/Zn alloy for decorations |
| Silver electrolytes | ||||
| ARGOL 2000 | 11.5 - 12.5 | bright white | 99.9%Ag | Jewelry, watches, decoration |
- Nickel electrolytes
Nickel layers are mostly used as diffusion barriers during the gold plating of copper and copper alloys or as an intermediate layer for tinning
- Bronze electrolytes
Bronze coatings – in white or yellow color tones – are used either as an allergy free nickel replacement or as a surface layer for decorative purposes. For technical applications the bronze layers are utilized for their good corrosion resistance and good brazing and soldering properties.
| Type of Electrolyte | pH-Range | Electrolyte temperature [°C] |
Current density [A/dm²] |
Yield [%] |
|---|---|---|---|---|
| Copper electrolytes | ||||
| Cyanide copper | 10 - 13 | 40 - 65 | 0 .5 - 4 | 70-95 |
| Acidic copper | <1 | 20 - 35 | 2 - 8 | <100 |
| Nickel electrolytes | ||||
| Watts nickel (Sulfate) |
3 - 5 | 40 - 70 | 3 - 10 | 95-97 |
| Sulfamate nickel | 3 - 4 | 30 - 60 | 5 - 20 | 95-97 |
| Tin electrolytes | ||||
| Acidic tin (Sulfate) | <1 | 18 - 25 | 1 - 3 | <100 |
| Alkaline tin | >10 | 75 - 80 | 2 - 17 | max.95 |
| Bronze electrolytes | ||||
| DODUBRONCE W | Strongly alkaline | 55 - 60 | 0.5 - 1.5 | |
| DODUBRONCE G | Strongly alkaline | 45 - 50 | 2 - 3.5 | |
| DODUBRONCE AF | Strongly alkaline | 58 - 62 | 0.5 - 1.5 | |
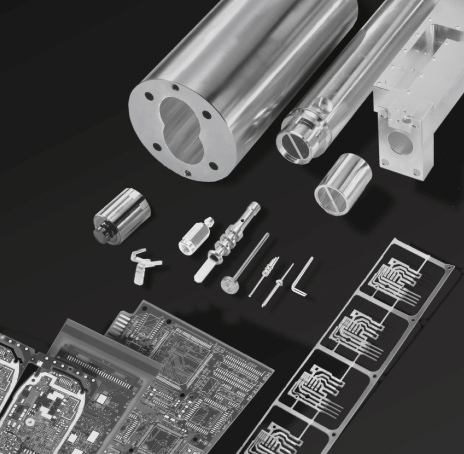
Electroplating of Parts
The complete or all-around electroplating of small mass produced parts, like contact springs, rivets or pins is usually done as mass plating in electroplating barrels of different shape. During the electroplating process the parts are continuously moved and mixed to reach a uniform coating.
Larger parts are frequently electroplated on racks either totally or by different masking techniques also partially. Penetrating the coating into the interior of drilled holes or tubes can be achieved with the use of special fixtures.
Electroplated Parts
- Materials
| Coatings | |
|---|---|
| Precious metals | Pure gold, hard gold (HV 150 – 250), palladium, palladium-nickel, rhodium, pure silver, hard silver (HV 130 – 160) |
| Non-precious metals | Copper, nickel, tin, tin alloys |
| Carrier materials | Copper, copper alloys, nickel, nickel alloys, iron, steel, aluminum, aluminum alloys, composite materials such as aluminum – silicon carbide |
- Coating thickness
| Precious metals: | 0.2 – 5 μm (typical layer thicknesses; for Ag also up to 25 μm) |
| Non-precious metals: | Up to approx. 20 μm |
| Tungsten | 0.5 N |
| Tolerances: | Strongly varying depending on the geometrical shape of parts(up to 50% at a defined measuring spot). It is recommended to specify a minimum value for the coating thickness at a defined measuring spot |
- Quality criteria
Besides others the following layer parameters are typically monitored in-process and documented:
- Coating thickness
- Adhesion strength
- Porosity
- Solderability
- Bonding property
- Contact resistance
These quality tests are performed according to industry standards, internal standards, and customer specifications resp.
Electroplating of Semi-finished Materials
The process for overall electroplating of strips, profiles and wires is mostly performed on continuously operating reel-to-reel equipment. The processing steps for the individual operations such as pre-cleaning, electroplating or rinsing, are following the same principles as those employed in parts electroplating.
The overall coating is usually applied for silver plating and tin coating of strips and wires. Compared to hard gold or palladium, these deposits are rather ductile, ensuring that during following stamping and forming operations, no cracks are generated in the electroplated layers.
Selective Electroplating
Since precious metals are rather expensive, it is necessary to perform the electroplating most economically and to coat only those areas that need the layers for functional purposes. This leads from overall plating to selective electroplating of strip material in continuous reel-to-reel processes. Depending on the final parts design and the end application, the processes can be applied to solid strip material, as well as pre-stamped and formed continuous strips or utilizing wire-formed or machined pins, which have been arranged as bandoliers attached to conductive metal strips.
The core part of selective precious metal electroplating is the actual electroplating cell. Inside the cell, the anode is arranged closely to the cathodic polarized material strip. Cathode screens or masks may be applied between the two, to focus the electrical field onto closely defined spots on the cathode strip.
Special high performance electrolytes are used in selective electroplating to reach short plating times and allow a high flow rate of the electrolyte for a fast electrolyte exchange in the actual coating area.
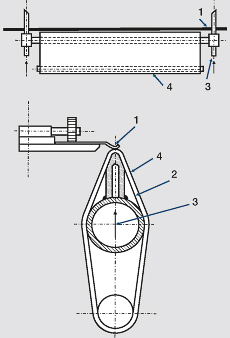
For a closely targeted electroplating of limited precious metal coating of contact springs, so-called brush-electroplating cells are employed (Figure 2). The “brush” or “tampon” consists of a roof shaped titanium metal part covered with a special felt-like material. The metal body has holes in defined spots, through which the electrolyte reaches the felt. Also located In the same spots is the anode, consisting of a fine platinum net. The pre-stamped and in the contact area pre-formed contact spring part is guided under a defined pressure over the electrolyte soaked felt material and gets wetted with the electrolyte. This allows the metal electroplating in highly selective spots.
For special applications, such as for example electronic component substrates, a dot shaped precious metal coating is required. This is achieved with two belt masks running synchronous to the carrier material. One of these two masks has windows, which are open to the spot areas targeted for precious metal plating coverage.
Summary of the processes for selective electroplating
- Immersion electroplating
Overall or selective electroplating of both sides of solid strips or pre-stamped parts in strip form
- Stripe electroplating
Stripe electroplating on solid strips through wheel cells or using masking techniques
- Selective electroplating
One-sided selective coating of solid, pre-stamped or metallically belt-linked strips by brush plating
- Spot electroplating
Electroplating in spots of solid strips with guide holes or pre-stamped parts in strip form
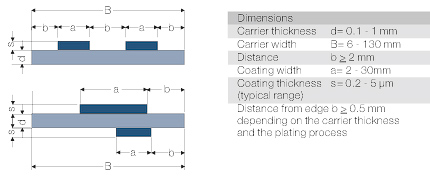
Typical examples of electroplated semi-finished materials (overall or selectively)
- Materials
| Type of Coatings | Coating Thickness | Remarks |
|---|---|---|
| Gold electrolytes | ||
| Pure gold Hard gold (AuCo 0.3) |
0.1 - 3 μm | In special cases up to 10 μm |
| Palladium-nickel (PdNi20) | 0.1 - 5 μm | Frequently with additional 0.2 μm AuCo 0.3 |
| Silver | 0.5 - 10 μm | In special cases up to 40 μm |
| Non-precious Metals | ||
| Nickel | 0.5 - 4 μm | Diffusion barrier especially for gold layers |
| Copper | 1 - 5 μm | Intermediate layer used in tinning of CuZn |
| Tin, tin alloys | 0.8 - 25 μm | materials |
- Carrier Materials
Copper, copper alloys, nickel, nickel alloys, stainless steel
- Dimensions and Tolerances
- Tolerances
| Coating thickness approx. | ± 10 % |
| Coating thickness and position | ± 0,5 mm |
- Quality Criteria
Mechanical properties and dimensional tolerances of the carrier materials follow the typical standards, i.e. DIN EN 1652 and 1654 for copper and copper alloys. Depending on the application, the following parameters are tested and recorded (see also: Electroplating of parts):
- Coating thickness
- Solderability
- Adhesion strength
- Bonding property
- Porosity
- Contact resistance
These quality tests are performed according to industrial standards, internal standards and customer specifications resp.