Difference between revisions of "Contact Carrier Materials"
(→5.1.2 Pure Copper) |
Doduco Admin (talk | contribs) (→High Cu Content Copper Alloys) |
||
| (149 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
The reliability and electrical life of contact systems in switching devices as well as in electromechanical and electronic components do not only depend on the contact material. The selection of the most suitable carrier material also plays an important role. | The reliability and electrical life of contact systems in switching devices as well as in electromechanical and electronic components do not only depend on the contact material. The selection of the most suitable carrier material also plays an important role. | ||
| − | The most frequently used ones are copper based materials. Depending on the application also materials based on nickel or multi-layer composite materials, | + | The most frequently used ones are copper based materials. Depending on the application, also materials based on nickel or multi-layer composite materials, |
such as thermo bimetals for example, are frequently used. For special applications in the medium and high voltage technology, as well as for springs | such as thermo bimetals for example, are frequently used. For special applications in the medium and high voltage technology, as well as for springs | ||
and snap discs for the information technology, iron or steel based materials are considered. These are however not included for the purpose of this data book. | and snap discs for the information technology, iron or steel based materials are considered. These are however not included for the purpose of this data book. | ||
| − | Various requirements based on the enduse of the contact components have to be met by carrier materials. Copper materials have to exhibit high electrical and thermal conductivity, good mechanical strength even at elevated temperatures | + | Various requirements based on the enduse of the contact components have to be met by carrier materials. Copper materials have to exhibit high electrical and thermal conductivity, good mechanical strength even at elevated temperatures and in addition, a sufficient high resistance against corrosion. If used as springs, the carrier materials also must have good elastic spring properties. Besides these, the materials must, depending on the manufacturing processes employed, also have good technological properties like ductility, to allow warm and cold forming, suitability for cutting and stamping and be capable to be welded, brazed or coated by electroplating. |
| − | ==5.1 Copper and Copper Alloys== | + | ==<!--5.1-->Copper and Copper Alloys== |
===Standards Overview=== | ===Standards Overview=== | ||
| − | Copper and copper alloys | + | Copper and copper alloys being used in electrical and electronic components are usually covered by national and international standards. DIN numbers the |
| − | materials by a prefix and/or a material number. The newer European standards (EN) refer to the material's usage products and also show a prefix and material number. For reference we also show in <xr id="tab:MaterialDesignations"/> the material designation according to UNS, the Unified Numbering System (USA). Other internationally used standard and material numbers include, among others, those issued by CDA (Copper Development Association, USA), and GB (Guo Biao – China). | + | materials by a prefix and/or a material number. The newer European standards (EN) refer to the material's usage products and also show a prefix and material number. For reference, we also show in (<xr id="tab:MaterialDesignations"/>) the material designation according to UNS, the Unified Numbering System (USA). Other internationally used standard and material numbers include, among others, those issued by CDA (Copper Development Association, USA), and GB (Guo Biao – China). |
| − | The most important EN as well as the US based and widely used ASTM standards covering the use of flat rolled copper and copper alloys in electrical contacts are: | + | The most important EN as well as the US based and widely used ASTM standards, covering the use of flat rolled copper and copper alloys in electrical contacts, are: |
{| class="twocolortable" style="text-align: left; font-size: 12px" | {| class="twocolortable" style="text-align: left; font-size: 12px" | ||
| Line 41: | Line 41: | ||
|- | |- | ||
|ASTM B 534-07 || Sec. for CuCoBe-Alloy and CuNiBe-Alloy Plate, Sheet, Strip, and Rolled Bar | |ASTM B 534-07 || Sec. for CuCoBe-Alloy and CuNiBe-Alloy Plate, Sheet, Strip, and Rolled Bar | ||
| + | |- | ||
|} | |} | ||
The above DIN EN standards replace in part or completely the older DIN standards DIN 1777, | The above DIN EN standards replace in part or completely the older DIN standards DIN 1777, | ||
DIN 17670, DIN 1751, DIN 1791. | DIN 17670, DIN 1751, DIN 1791. | ||
| − | ===5.1.2 Pure Copper=== | + | ===<!--5.1.2-->Pure Copper=== |
| − | Copper is used in electrical engineering mostly because of its high electrical conductivity<ref>As units for electrical conductivity MS/m and m/Ω.mm<sup>2</sup> are commonly used. Frequently – and mostly in North America – the % IACS value (International Annealed Copper Standard) is also used, where 100% is equivalent to 58 MS/m or m/Ωmm<sup>2</sup> .For the description of mechanical strength properties the units of N/mm<sup>2</sup> or MPa are most commonly used: | + | Copper is used in electrical engineering, mostly because of its high electrical conductivity<ref>As units for electrical conductivity MS/m and m/Ω.mm<sup>2</sup> are commonly used. Frequently – and mostly in North America – the % IACS value (International Annealed Copper Standard) is also used, where 100% is equivalent to 58 MS/m or m/Ωmm<sup>2</sup> .For the description of mechanical strength properties the units of N/mm<sup>2</sup> or MPa are most commonly used: |
1 MS/m = 1 m/Ωmm<sup>2</sup> | 1 MS/m = 1 m/Ωmm<sup>2</sup> | ||
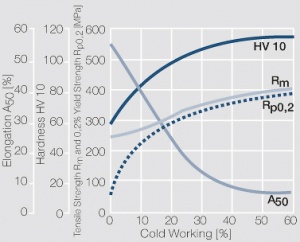
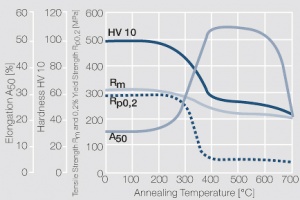
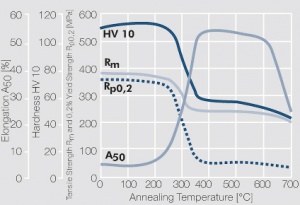
| − | 1 MPa = 1 N/mm<sup>2</sup></ref> which with 58 MS/m (or m/Ωmm²) is only slightly below that of silver. Other advantages of copper are its high thermal conductivity, corrosion resistance | + | 1 MPa = 1 N/mm<sup>2</sup></ref> which with 58 MS/m (or m/Ωmm²) is only slightly below that of silver. Other advantages of copper are its high thermal conductivity, corrosion resistance and its good ductility. The work hardening properties of ETP copper is illustrated in (<xr id="fig:Strain hardening of Cu-ETP by cold working" />). The increase in strength achieved by cold working can be reversed easily by subsequent annealing. The softening properties are strongly dependent on the preceding cold working percentage (<xr id="fig:Softening of Cu-ETP after annealing for 3hrs after 25% cold working"/> and <xr id="fig:Softening of Cu-ETP after annealing for 3hrs after 50% cold working"/><!--5.3-->). |
The purity of technically pure and un-alloyed copper used for electrical applications depends on the type used and ranges between > 99.90 and 99.95 | The purity of technically pure and un-alloyed copper used for electrical applications depends on the type used and ranges between > 99.90 and 99.95 | ||
wt%. The copper types are designated mainly by their oxygen content as oxygen containing, oxygen-free, and de-oxidized with phosphorus as | wt%. The copper types are designated mainly by their oxygen content as oxygen containing, oxygen-free, and de-oxidized with phosphorus as | ||
| − | described in DIN EN 1652 | + | described in DIN EN 1652 (<xr id="tab:MaterialDesignations"/> and <xr id="tab:Composition of Some Pure Copper Types"/><!--5.2-->). <xr id="tab:Physical Properties of Some Copper Types"/><!--Tables 5.3.--> and <xr id="tab:Mechanical Properties of Some Copper Types"/><!--5.4--> show the physical and mechanical properties of these copper materials. According to these, Cu-ETP, Cu-OFE and Cu-HCP are the types of copper for which minimum values for the electrical conductivity are guaranteed. |
Cu-ETP is less suitable for welding or for brazing in reducing atmosphere because of the oxygen content (danger of hydrogen embrittlement). | Cu-ETP is less suitable for welding or for brazing in reducing atmosphere because of the oxygen content (danger of hydrogen embrittlement). | ||
| − | Cu-HCP, Cu-DLP, and Cu-DHP are oxygen free copper types de-oxidized with different phosphorus contents. With increasing phosphorus content the | + | Cu-HCP, Cu-DLP, and Cu-DHP are oxygen free copper types, de-oxidized with different phosphorus contents. With increasing phosphorus content, the |
electrical conductivity decreases. Cu-OFE, also called OFHC copper, is free of oxygen and also free of de-oxidizing compounds. | electrical conductivity decreases. Cu-OFE, also called OFHC copper, is free of oxygen and also free of de-oxidizing compounds. | ||
<figtable id="tab:MaterialDesignations"> | <figtable id="tab:MaterialDesignations"> | ||
| − | '''Table 5.1: Material Designations of Some Copper Types''' | + | <caption>'''<!--Table 5.1:-->Material Designations of Some Copper Types'''</caption> |
<table class="twocolortable" border="1" cellspacing="0" style="border-collapse:collapse"> | <table class="twocolortable" border="1" cellspacing="0" style="border-collapse:collapse"> | ||
| − | + | ||
<tr> | <tr> | ||
<th>WerkstMaterialEN-Designation</th><th>EN-Number</th><th>DIN-Designation</th><th>DIN-Number</th><th>UNS</th></tr> | <th>WerkstMaterialEN-Designation</th><th>EN-Number</th><th>DIN-Designation</th><th>DIN-Number</th><th>UNS</th></tr> | ||
| Line 86: | Line 87: | ||
| − | <figtable id="tab: | + | <figtable id="tab:Composition of Some Pure Copper Types"> |
| − | '''Table 5.2: Composition of Some Pure Copper Types''' | + | <caption>'''<!--Table 5.2:-->Composition of Some Pure Copper Types'''</caption> |
{| class="twocolortable" style="text-align: left; font-size: 12px" | {| class="twocolortable" style="text-align: left; font-size: 12px" | ||
| Line 146: | Line 147: | ||
| − | <figtable id="tab: | + | <figtable id="tab:Physical Properties of Some Copper Types"> |
| − | '''Table 5.3: Physical Properties of Some Copper Types''' | + | <caption>'''<!--Table 5.3:-->Physical Properties of Some Copper Types'''</caption> |
| − | <table | + | <table class="twocolortable"> |
| + | <tr><th><p class="s3">Material</p></th><th ><p class="s3">Density</p></th><th colspan="2"><p class="s3">Electrical</p><p class="s3">Conductivityt</p></th><th ><p class="s3">Electrical Conductivity</p></th><th><p class="s3">Thermal</p><p class="s3">Conductivity</p></th> | ||
| + | <th><p class="s3">Coeff. of</p><p class="s3">Linear Thermal Expansion</p></th> | ||
| + | <th><p class="s3">Modulus</p><p class="s3">of</p><p class="s3">Elasticity</p></th><th><p class="s3">Softening</p><p class="s3">Temperatur (approx.</p>10% loss in strength)</th><th><p class="s3">Melting</p><p class="s3">Temperature</p></th></tr> | ||
| + | <tr><th><p class="s3">EN- Designation</p></th> | ||
| + | <th >[g/cm³]</th><th>[MS/m]</th><th>[% IACS]</th><th>[μΩ· cm]</th><th>[W/(m· K)]</th> | ||
| + | <th>[10<sup>-6</sup>/K]</th><th>[GPa]</th><th>[°C]</th><th>[°C]</th></tr> | ||
| + | <tr><td><p class="s3">Cu-ETP</p></td><td><p class="s3">8.94</p></td> | ||
| + | <td>≥58</td> | ||
| + | <td><p class="s3">100</p></td><td><p class="s3">1.72</p></td><td><p class="s3">390</p></td><td><p class="s3">17.7</p></td><td><p class="s3">127</p></td><td><p class="s3">ca. 220</p></td><td><p class="s3">1083</p></td></tr><tr><td><p class="s3">Cu-OF</p></td><td><p class="s3">8.94</p></td><td>≥58</td><td><p class="s3">100</p></td><td><p class="s3">1.72</p></td><td><p class="s3">394</p></td><td><p class="s3">17.7</p></td><td><p class="s3">127</p></td><td><p class="s3">ca. 220</p></td><td><p class="s3">1083</p></td></tr><tr><td><p class="s3">Cu-HCP</p></td><td><p class="s3">8.94</p></td><td>≥54</td><td><p class="s3">93</p></td><td><p class="s3">1.85</p></td><td><p class="s3">380</p></td><td><p class="s3">17.7</p></td><td><p class="s3">127</p></td><td><p class="s3">ca. 220</p></td><td><p class="s3">1083</p></td></tr><tr><td><p class="s3">Cu-DLP</p></td><td><p class="s3">8.94</p></td><td><p class="s3">52</p></td><td><p class="s3">90</p></td><td><p class="s3">1.92</p></td><td><p class="s3">350</p></td><td><p class="s3">17.7</p></td><td><p class="s3">132</p></td><td><p class="s3">ca. 220</p></td><td><p class="s3">1083</p></td></tr><tr><td><p class="s3">Cu-DHP</p></td><td><p class="s3">8.94</p></td><td>≥46</td><td><p class="s3">80</p></td><td><p class="s3">2.17</p></td><td><p class="s3">310</p></td><td><p class="s3">17.6</p></td><td><p class="s3">132</p></td><td><p class="s3">ca. 220</p></td><td><p class="s3">1083</p></td></tr></table> | ||
</figtable> | </figtable> | ||
| − | <figtable id="tab: | + | <figtable id="tab:Mechanical Properties of Some Copper Types"> |
| − | '''Table 5.4: Mechanical Properties of Some Copper Types''' | + | <caption>'''<!--Table 5.4:-->Mechanical Properties of Some Copper Types'''</caption> |
<table class="twocolortable" border="1" cellspacing="0" style="border-collapse:collapse"> | <table class="twocolortable" border="1" cellspacing="0" style="border-collapse:collapse"> | ||
| Line 165: | Line 175: | ||
Cu-ETP<br> Cu-OF <br> Cu-HCP <br>Cu-DLP<br> Cu-DHP</td> | Cu-ETP<br> Cu-OF <br> Cu-HCP <br>Cu-DLP<br> Cu-DHP</td> | ||
<td>R220</td><td>220 - 260</td> | <td>R220</td><td>220 - 260</td> | ||
| − | <td>& | + | <td>≤140</td><td>≥33</td><td>40 - 65</td> |
</tr><tr> | </tr><tr> | ||
<td>R240</td><td>240 - 300</td> | <td>R240</td><td>240 - 300</td> | ||
| − | <td>& | + | <td>≥180</td> |
| − | <td | + | <td>≥8</td><td>65 - 95</td> |
</tr><tr> | </tr><tr> | ||
| − | <td>R290</td><td>290 - 360</td><td>& | + | <td>R290</td><td>290 - 360</td><td>≥250</td><td>≥4</td><td>90 - 110</td> |
</tr><tr> | </tr><tr> | ||
| − | <td>R360</td><td>& | + | <td>R360</td><td>≥360</td><td>≥320</td><td>≥2</td> |
| − | <td>& | + | <td>≥110</td></tr> |
</table> | </table> | ||
</figtable> | </figtable> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<div class="multiple-images"> | <div class="multiple-images"> | ||
| − | <figure id="fig: | + | <figure id="fig:Strain hardening of Cu-ETP by cold working"> |
[[File:Strain hardening of Cu ETP by cold working.jpg|left|thumb|<caption>Strain hardening of Cu-ETP by cold working</caption>]] | [[File:Strain hardening of Cu ETP by cold working.jpg|left|thumb|<caption>Strain hardening of Cu-ETP by cold working</caption>]] | ||
</figure> | </figure> | ||
| − | <figure id="fig: | + | <figure id="fig:Softening of Cu-ETP after annealing for 3hrs after 25% cold working"> |
[[File:Softening of Cu ETP after annealing.jpg|left|thumb|<caption>Softening of Cu-ETP after annealing for 3hrs after 25% cold working</caption>]] | [[File:Softening of Cu ETP after annealing.jpg|left|thumb|<caption>Softening of Cu-ETP after annealing for 3hrs after 25% cold working</caption>]] | ||
</figure> | </figure> | ||
| − | <figure id="fig: | + | <figure id="fig:Softening of Cu-ETP after annealing for 3hrs after 50% cold working"> |
[[File:Softening of Cu ETP after annealing 50.jpg|left|thumb|<caption>Softening of Cu-ETP after annealing for 3hrs after 50% cold working</caption>]] | [[File:Softening of Cu ETP after annealing 50.jpg|left|thumb|<caption>Softening of Cu-ETP after annealing for 3hrs after 50% cold working</caption>]] | ||
</figure> | </figure> | ||
| Line 201: | Line 204: | ||
<div class="clear"></div> | <div class="clear"></div> | ||
| − | ===5.1.3 High Cu Content Copper Alloys=== | + | ===<!--5.1.3-->High Cu Content Copper Alloys=== |
| + | |||
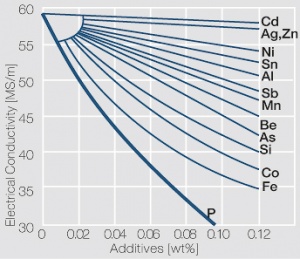
| + | The high Cu content alloy materials are closest in their properties to pure copper materials. By defined addition of small amounts of alloying elements, it is possible to increase the mechanical strength and especially the softening temperature of copper and at the same time decrease the electrical conductivity only insignificantly (<xr id="fig:Influence of small additions on the electrical conductivity of copper"/><!--(Fig. 5.4)-->). Silver, iron, tin, zinc, nickel, chromium, zirconium, silicon and titanium are used. Usually, the additive amounts are significantly below 3 wt%. This group of materials consists of mixed crystal as well as precipitation hardening alloys. The precipitation hardening copper-beryllium and copper-chromium-zirconium materials are decribed later in a separate section. | ||
| − | + | <figure id="fig:Influence of small additions on the electrical conductivity of copper"> | |
| + | [[File:Influence of small additions on the electrical conductivity of copper.jpg|right|thumb|Figure 4: Influence of small additions on the electrical conductivity of copper]] | ||
| + | </figure> | ||
| − | From the large number of high-Cu alloys only the properties of selected ones are covered here | + | From the large number of high-Cu alloys, only the properties of selected ones are covered here (<xr id="tab:Physical Properties of Selected High Cu Content Copper Alloys"/><!--(Tab. 5.5)--> and <xr id="tab:Mechanical Properties of Selected High Cu Content Copper Alloys"/><!--(Tab. 5.6)-->). Some of these materials are not included in the EN standards system. |
| − | The low alloyed materials CuAg0.1 and CuCd1 are mostly used as overhead drive cables where they have to meet sustained loads at elevated temperatures without softening. | + | The low alloyed materials CuAg0.1 and CuCd1 are mostly used as overhead drive cables, where they have to meet sustained loads at elevated temperatures without softening. |
| − | The materials CuFe0.1 and CuSn0.15 have a high electrical conductivity. The mechanical strength of both is relatively low but stays almost constant at temperatures up to 400°C. | + | The materials CuFe0.1 and CuSn0.15 have a high electrical conductivity. The mechanical strength of both is relatively low but stays almost constant at temperatures up to 400°C. They are used as substrates for power semiconductors and also as carriers for stationary contacts in higher energy switchgear. |
| − | switchgear. | ||
| − | CuFe2 is a material exhibiting high electrical conductivity and good formability. During an annealing process Fe-rich precipitations are formed in the | + | CuFe2 is a material exhibiting high electrical conductivity and good formability. During an annealing process, Fe-rich precipitations are formed in the Cu matrix, which changes the mechanical properties very little but increases the electrical conductivity significantly. Besides being used as a contact carrier material in switching devices, this material has broader applications in automotive connectors and as a substrate in the semiconductor technology. |
| − | CuNi2Si has high mechanical strength, good formability | + | CuNi2Si has high mechanical strength, good formability and at the same time high electrical conductivity. This combination of advantageous properties is achieved by a defined finely dispersed precipitation of nickel silicides. CuNi2Si is used mainly in the form of stamped and formed parts in thermally stressed electromechanical components for automotive applications. |
CuSn1CrNiTi and CuCrSiTi are advanced developments of the Cu-Cr-Ti precipitation materials with fine intermetallic dispersions. The material | CuSn1CrNiTi and CuCrSiTi are advanced developments of the Cu-Cr-Ti precipitation materials with fine intermetallic dispersions. The material | ||
CuNi1Co1Si also belongs into this family and has properties similar to the low alloyed CuBe materials. | CuNi1Co1Si also belongs into this family and has properties similar to the low alloyed CuBe materials. | ||
| − | + | <br style="clear:both;"/> | |
| − | 2 | + | <figtable id="tab:Physical Properties of Selected High Cu Content Copper Alloys"> |
| + | <caption>'''<!--Tab. 5.5-->Physical Properties of Selected High Cu Content Copper Alloys'''</caption> | ||
| + | |||
| + | {| class="twocolortable" style="text-align: left; font-size: 12px" | ||
| + | |- | ||
| + | !Material/<br />Designation<br />EN UNS | ||
| + | !Composition | ||
| + | !Density<br />[g/cm<sup>3</sup>] | ||
| + | !colspan="2" style="text-align:center"|Electrical<br />Conductivity | ||
| + | !Electrical<br />Resistivity<br />[μΩ·cm] | ||
| + | !Thermal<br />Conductivity<br />[W/(m·K)] | ||
| + | !Coeff. of Linear<br />Thermal<br />Expansion<br />[10<sup>-6</sup>/K] | ||
| + | !Modulus of<br />Elasticity<br />[GPa] | ||
| + | !Softening Temperature<br />(approx. 10% loss in<br />strength)<br />[°C] | ||
| + | !Melting<br />Temp Range<br />[°C] | ||
| + | |- | ||
| + | ! | ||
| + | ! | ||
| + | ! | ||
| + | ![MS/m] | ||
| + | ![% IACS] | ||
| + | ! | ||
| + | ! | ||
| + | ! | ||
| + | ! | ||
| + | ! | ||
| + | ! | ||
| + | |- | ||
| + | |CuAg 0,1<br />CW 013A | ||
| + | |Ag 0.08-0.12<br />Cu Rest | ||
| + | |8.89 | ||
| + | |56 | ||
| + | |97 | ||
| + | |1.8 | ||
| + | |380 | ||
| + | |17.7 | ||
| + | |126 | ||
| + | | | ||
| + | |1082 | ||
| + | |- | ||
| + | |CuFe0,1P<br />not standardized<br />C19210 | ||
| + | |Fe 0.05-0.015<br />P 0.025-0.04<br />Cu Rest | ||
| + | |8.89 | ||
| + | |53 | ||
| + | |91 | ||
| + | |1.9 | ||
| + | |350 | ||
| + | |17.0 | ||
| + | |130 | ||
| + | |ca. 280 | ||
| + | |1080 | ||
| + | |- | ||
| + | |CuSn0,15<br />CW117C<br />C14415 | ||
| + | |Sn 0.1-0.15<br />Zn 0.1<br />Cu Rest | ||
| + | |8.93 | ||
| + | |51 | ||
| + | |88 | ||
| + | |2.0 | ||
| + | |350 | ||
| + | |18.0 | ||
| + | |130 | ||
| + | |ca. 280 | ||
| + | |1060 | ||
| + | |- | ||
| + | |CuFe2P<br />CW107C<br />C19400 | ||
| + | |Fe 2.1-2.6<br />P 0.015-0.15<br />Zn 0.05-0.2<br />Cu Rest | ||
| + | |8.91 | ||
| + | |37 | ||
| + | |64 | ||
| + | |2.7 | ||
| + | |260 | ||
| + | |17.6 | ||
| + | |125 | ||
| + | |ca. 380 | ||
| + | |1084 - 1090 | ||
| + | |- | ||
| + | |CuNi2Si<br />CW111C<br />C70260 | ||
| + | |Ni 1.6-2.5<br />Si 0.4-0.8<br />Fe 0.2<br />Cu Rest | ||
| + | |8.80 | ||
| + | |23 | ||
| + | |40 | ||
| + | |4.3 | ||
| + | |200 | ||
| + | |17.0 | ||
| + | |130 | ||
| + | |ca. 430 | ||
| + | | | ||
| + | |- | ||
| + | |CuSn1CrNiTi<br />not standardized<br />C18090 | ||
| + | |Sn 0.6<br />Ni 0.4<br />Cr 0.3<br />Ti 0.3<br />Cu Rest | ||
| + | |8.87 | ||
| + | |35 | ||
| + | |60 | ||
| + | |2.9 | ||
| + | |240 | ||
| + | |17.6 | ||
| + | |133 | ||
| + | |ca. 480 | ||
| + | |1025 - 1074 | ||
| + | |- | ||
| + | |CuNi1Co1Si<br />not standardized<br />C70350 | ||
| + | |Ni 1.5<br />Co 1.1<br />Si 0.6<br />Cu Rest | ||
| + | |8.82 | ||
| + | |29 | ||
| + | |50 | ||
| + | |3.4 | ||
| + | |200 | ||
| + | |17.6 | ||
| + | |131 | ||
| + | |ca. 400 | ||
| + | | | ||
| + | |- | ||
| + | |CuCrSiTi<br />not standardized<br />C18070 | ||
| + | |Cr 0.3<br />Ti 0.1<br />Si 0.02<br />Cu Rest | ||
| + | |8.88 | ||
| + | |45 | ||
| + | |78 | ||
| + | |2.2 | ||
| + | |310 | ||
| + | |18.0 | ||
| + | |138 | ||
| + | |ca. 430 | ||
| + | | | ||
| + | |} | ||
| + | </figtable> | ||
| − | |||
| − | |||
| − | + | <figtable id="tab:Mechanical Properties of Selected High Cu Content Copper Alloys"> | |
| + | <caption>'''<!--Table 5.6:-->Mechanical Properties of Selected High Cu Content Copper Alloys'''</caption> | ||
| − | + | {| class="twocolortable" style="text-align: left; font-size: 12px" | |
| − | + | |- | |
| − | + | !Material | |
| − | </ | + | !Hardness<br />Condition |
| + | !Tensile Strength R<sub>m</sub><br />[MPa] | ||
| + | !0,2% YieldStrength<br />R<sub>p02</sub><br />[MPa] | ||
| + | !Elongation<br />A<sub>50</sub><br />[%] | ||
| + | !Vickers<br />Hardness<br />HV | ||
| + | !Bend Radius<sup>1)</sup><br />perpendicular to<br />rolling direction | ||
| + | !Bend Radius<sup>1)</sup><br />parallel to<br />rolling direction | ||
| + | !Spring Bending<br />Limit σ<sub>FB</sub><br />[MPa] | ||
| + | !Spring Fatigue<br />Limit σ<sub>BW</sub><br />[MPa] | ||
| + | |- | ||
| + | |CuAg0,10 | ||
| + | |R 200<br />R 360 | ||
| + | |200 - 250<br />360 | ||
| + | |120<br />320 | ||
| + | |> 40<br />> 3 | ||
| + | |40<br />90 | ||
| + | |0 x t<br />0.5 x t | ||
| + | |0 x t<br />0.5 x t | ||
| + | |240 | ||
| + | |120 | ||
| + | |- | ||
| + | |CuFe0,1P | ||
| + | |R 300<br />R 360<br />R 420 | ||
| + | |300 - 380<br />360 - 440<br />420 - 500 | ||
| + | |> 260<br />> 300<br />> 350 | ||
| + | |> 10<br />> 3<br />> 2 | ||
| + | |80 - 110<br />110 - 130<br />120 - 150 | ||
| + | |0 x t<br />0.5 x t<br />1.5 x t | ||
| + | |0 x t<br />0.5 x t<br />1.5 x t | ||
| + | |250 | ||
| + | |160 | ||
| + | |- | ||
| + | |CuSn0,15 | ||
| + | |R 250<br />R 300<br />R 360<br />R 420 | ||
| + | |250 - 320<br />300 - 370<br />360 - 430<br />420 - 490 | ||
| + | |> 200<br />> 250<br />> 300<br />> 350 | ||
| + | |> 9<br />> 4<br />> 3<br />> 2 | ||
| + | |60 - 90<br />85 - 110<br />105 - 130<br />120 - 140 | ||
| + | |0 x t<br />0 x t<br />0 x t<br />1 x t | ||
| + | |0 x t<br />0 x t<br />0 x t<br />1 x t | ||
| + | |250 | ||
| + | |160 | ||
| + | |- | ||
| + | |CuFe2P | ||
| + | |R 370<br />R 420<br />R 470<br />R 520 | ||
| + | |370 - 430<br />420 - 480<br />470 - 530<br />520 - 580 | ||
| + | |> 300<br />> 380<br />> 430<br />> 470 | ||
| + | |> 6<br />> 4<br />> 4<br />> 3 | ||
| + | |115 - 135<br />130 - 150<br />140 - 160<br />150 - 170 | ||
| + | |0 x t<br />0.5 x t<br />0.5 x t<br />1 x t | ||
| + | |0 x t<br />0.5 x t<br />0.5 x t<br />1 x t | ||
| + | |340 | ||
| + | |200 | ||
| + | |- | ||
| + | |CuNi2Si | ||
| + | |R 430<br />R 510<br />R 600 | ||
| + | |430 - 520<br />510 - 600<br />600 - 680 | ||
| + | |> 350<br />> 450<br />> 550 | ||
| + | |> 10<br />> 7<br />> 5 | ||
| + | |125 - 155<br />150 - 180<br />180 - 210 | ||
| + | |0 x t<br />0 x t<br />1 x t | ||
| + | |0 x t<br />0 x t<br />1 x t | ||
| + | |500 | ||
| + | |230 | ||
| + | |- | ||
| + | |CuSn1CrNiTi | ||
| + | |R 450<br />R 540<br />R 620 | ||
| + | |450 - 550<br />540 - 620<br />620 - 700 | ||
| + | |> 350<br />> 450<br />> 520 | ||
| + | |> 9<br />> 6<br />> 3 | ||
| + | |130 - 170<br />160 - 200<br />180 - 220 | ||
| + | |0.5 x t<br />1 x t<br />3 x t | ||
| + | |0.5 x t<br />2 x t<br />6 x t | ||
| + | |530 | ||
| + | |250 | ||
| + | |- | ||
| + | |CuNi1Co1Si | ||
| + | |R 800<br />R 850 | ||
| + | |> 800<br />> 850 | ||
| + | |> 760<br />> 830 | ||
| + | |> 4<br />> 1 | ||
| + | |> 260<br />> 275 | ||
| + | |0.5 x t<br />1.5 x t | ||
| + | |1.5 x t<br />2.5 x t | ||
| + | | | ||
| + | | | ||
| + | |- | ||
| + | |CuCrSiTi | ||
| + | |R 400<br />R 460<br />R 530 | ||
| + | |400 - 480<br />460 - 540<br />530 - 610 | ||
| + | |> 300<br />> 370<br />> 460 | ||
| + | |> 8<br />> 5<br />> 2 | ||
| + | |120 - 150<br />140 - 170<br />150 - 190 | ||
| + | |0 x t<br />0.5 x t<br />1 x t | ||
| + | |0 x t<br />0.5 x t<br />1 x t | ||
| + | |400 | ||
| + | |220 | ||
| + | |} | ||
| + | </figtable> | ||
| + | <sup>1)</sup> t: Strip thickness max. 0.5 mm | ||
| + | |||
| + | These newer copper based materials optimize properties, such as electrical conductivity, mechanical strength and relaxation, which are custom tailored to specific applications. Typical uses include contact springs for relays, switches and connectors. | ||
| − | ===5.1.4 Naturally Hard Copper Alloys=== | + | ===<!--5.1.4-->Naturally Hard Copper Alloys=== |
| − | Alloys like brasses (CuZn), tin bronzes (CuSN) | + | Alloys like brasses (CuZn), tin bronzes (CuSN) and German silver (CuNiZn), for which the required hardness is achieved by cold working, are defined as naturally hard alloys. Included in this group are also the silver bronzes (CuAg) with 2 – 6 wt% of Ag. |
Main Articel: [[Naturally Hard Copper Alloys| Naturally Hard Copper Alloys]] | Main Articel: [[Naturally Hard Copper Alloys| Naturally Hard Copper Alloys]] | ||
| − | + | ===<!--5.1.5-->Other Naturally Hard Copper Alloys=== | |
| − | ===5.1.5 Other Naturally Hard Copper Alloys=== | ||
Main Articel: [[Other Naturally Hard Copper Alloys| Other Naturally Hard Copper Alloys]] | Main Articel: [[Other Naturally Hard Copper Alloys| Other Naturally Hard Copper Alloys]] | ||
| − | ===5.1.6 Precipitation Hardening Copper Alloys=== | + | ===<!--5.1.6-->Precipitation Hardening Copper Alloys=== |
| − | Besides the naturally hard copper materials precipitation hardening copper alloys play also an important role as carrier materials for electrical contacts. By means of a suitable heat treatment finely dispersed precipitations of a second phase can be achieved which | + | Besides the naturally hard copper materials, precipitation hardening copper alloys play also an important role as carrier materials for electrical contacts. By means of a suitable heat treatment, finely dispersed precipitations of a second phase can be achieved, which increases the mechanical strength of these copper alloys significantly. |
Main Articel: [[Precipitation Hardening Copper Alloys| Precipitation Hardening Copper Alloys]] | Main Articel: [[Precipitation Hardening Copper Alloys| Precipitation Hardening Copper Alloys]] | ||
| + | ===<!--5.1.7-->Application Properties for the Selection of Copper Alloys=== | ||
| − | + | Important for the usage as spring contact components are, besides mechanical strength and electrical conductivity, mainly the typical spring properties such as the maximum spring bending limit and the fatigue strength as well as the bendability. During severe thermal stressing the behavior of spring materials is determined by their softening and relaxation. The following briefly describes these material properties. | |
| − | |||
| − | Important for the usage as spring contact components are besides mechanical strength and electrical conductivity mainly the typical spring properties such as the maximum spring bending limit and the fatigue strength as well as the bendability. During severe thermal stressing the behavior of spring materials is determined by their softening and relaxation. The following briefly describes these material properties. | ||
Main Articel: [[Application Properties for the Selection of Copper Alloys| Application Properties for the Selection of Copper Alloys]] | Main Articel: [[Application Properties for the Selection of Copper Alloys| Application Properties for the Selection of Copper Alloys]] | ||
| + | ===<!--5.1.8-->Selection Criteria for Copper-Based Materials=== | ||
| − | + | The selection of copper-based materials from the broad spectrum of available materials must be based on the requirements of the application. First, an application profile should be established which can be used to define the material properties. Usually there is however no single material that can fulfill all requirements to the same degree. A compromise must be found as for example between electrical conductivity and spring properties. | |
| − | |||
| − | The selection of copper-based materials from the broad spectrum of available materials must be based on the requirements of the application. First an | ||
| − | application profile should be established which can be used to define the material properties. Usually there is however no single material that can fulfill all requirements to the same degree. A compromise must be found as for example between electrical conductivity and spring properties. | ||
If current carrying capability is the key requirement, mechanical strength may have to be sacrificed as for example in carrier parts for stationary contacts. In this case, depending on the current level, pure copper or low alloyed copper materials such as CuSn0.15, or for economic reasons CuZn30, may be suitable. | If current carrying capability is the key requirement, mechanical strength may have to be sacrificed as for example in carrier parts for stationary contacts. In this case, depending on the current level, pure copper or low alloyed copper materials such as CuSn0.15, or for economic reasons CuZn30, may be suitable. | ||
| − | For spring contact components the interdependent relations between electrical conductivity and fatigue strength, or electrical conductivity and relaxation behavior are of main importance. The first case is critical for higher load relay springs. CuAg2 plays an important role for these applications. The latter is critical for components that are exposed to continuing high mechanical stresses like for example in connectors. The spring force must stay close to constant over the expected life time of the parts even at elevated temperatures from the environment or current carrying. In this case the relaxation behavior of the copper materials which may cause a decrease in spring force over time must be considered. Besides this easy forming during manufacturing must be possible; this means that bending operations can also be performed at high mechanical strength values. | + | For spring contact components, the interdependent relations between electrical conductivity and fatigue strength, or electrical conductivity and relaxation behavior are of main importance. The first case is critical for higher load relay springs. CuAg2 plays an important role for these applications. The latter is critical for components that are exposed to continuing high mechanical stresses like for example in connectors. The spring force must stay close to constant over the expected life time of the parts, even at elevated temperatures from the environment or current carrying. In this case, the relaxation behavior of the copper materials, which may cause a decrease in spring force over time, must be considered. Besides this, easy forming during manufacturing must be possible; this means that bending operations can also be performed at high mechanical strength values. |
| − | The increasing requirements on spring components in connectors, especially for use in automotive applications, such as higher surrounding temperatures, increased reliability | + | The increasing requirements on spring components in connectors, especially for use in automotive applications, such as higher surrounding temperatures, increased reliability and the trend towards miniaturization led to a change of materials from traditionally CuZn30 and CuSn4 to CuNiSi alloys, for example. These CuNiSi alloys and the newer heavy duty copper alloys like CuNi1Co1, are significantly improved with regards to mechanical strength, relaxation behavior and electrical conductivity. |
| − | ==5.2 Nickel and Nickel Alloys== | + | ==<!--5.2-->Nickel and Nickel Alloys== |
| − | ===5.2.1 Technical Grade Pure Nickel=== | + | ===<!--5.2.1-->Technical Grade Pure Nickel=== |
| − | Technical grade pure nickel commonly contains 99.0 to 99.8 wt% Ni and up to 1 wt% Co. Other ingredients are iron and manganese | + | Technical grade pure nickel commonly contains 99.0 to 99.8 wt% Ni and up to 1 wt% Co. Other ingredients are iron and manganese (<xr id="tab:Physical Properties of Nickel and Nickel Alloys"/><!--(Tab. 5.21)--> and <xr id="tab:Mechanical Properties of Nickel and Nickel Alloys"/><!--(Tab. 5.22)-->). Work hardening and softening behavior of nickel are shown in [[#figures11|(Figs. 5 – 6)]]<!--Figs. 5.45 and 5.46-->. |
| − | One of the significant properties of nickel is its modulus of elasticity which is almost twice as high as that of copper. At temperatures up to 345°C nickel is ferro-magnetic. | + | One of the significant properties of nickel is its modulus of elasticity, which is almost twice as high as that of copper. At temperatures up to 345°C nickel is ferro-magnetic. |
| − | Nickel has a high corrosion resistance, is very ductile | + | Nickel has a high corrosion resistance, is very ductile and easy to weld and clad. It is of great importance as a backing material for multiple layer weld profiles. In addition, nickel is used as an intermediate layer for thin claddings, acting as an effective diffusion barrier between copper containing carrier materials and gold or palladium-based contact materials. |
Because of the always present thin oxide layer on its surface, nickel is not suitable as a contact material for switching contacts. | Because of the always present thin oxide layer on its surface, nickel is not suitable as a contact material for switching contacts. | ||
| − | |||
| − | |||
| − | + | <div class="multiple-images"> | |
| − | [[File: | + | <figure id="fig:Strain hardening of technical pure nickel by cold working"> |
| + | [[File:Strain hardening of technical pure nickel by cold working.jpg|right|thumb|Figure 5: Strain hardening of technical pure nickel by cold working]] | ||
| + | </figure> | ||
| − | = | + | <figure id="fig:Softening of technical grad nickel after annealing for 3 hrs"> |
| + | [[File:Softening of technical grad nickel after annealing for 3 hrs.jpg|right|thumb|Figure 6: Softening of technical grad nickel after annealing for 3 hrs after 50% cold working]] | ||
| + | </figure> | ||
| + | </div> | ||
| + | <div class="clear"></div> | ||
| − | + | ===<!--5.2.2-->Nickel Alloys=== | |
| − | + | Because of its low electrical conductivity, NiCu30Fe is besides pure Ni and CuNi alloys the most widely used backing material for weldable contact components. With 1 – 2 wt% additives of Fe as well as 0.5 – 1 wt% Mn and Co, the mechanical strength of the binary alloy NiCu30 can be increased. | |
| − | + | The strength values of NiCu30Fe are significantly higher than those of the copper rich CuNi alloys [[#figures12|(Figs. 7 – 8)]]<!--(Figs. 5.47 and 5.48)-->. The good spring properties and thermal stability of NiCu30Fe make it a suitable material for the use as thermally stressed contact springs. | |
| − | |||
| − | + | <div class="multiple-images"> | |
| − | [[File: | + | <figure id="fig:Strain hardening of NiCu30Fe by cold working"> |
| + | [[File:Strain hardening of NiCu30Fe by cold working.jpg|right|thumb|Figure 7: Strain hardening of NiCu30Fe by cold working]] | ||
| + | </figure> | ||
| − | + | <figure id="fig:Softening of NiCu30Fe after annealing for 0.5 hrs"> | |
| + | [[File:Softening of NiCu30Fe after annealing for 0.5 hrs.jpg|right|thumb|Figure 8: Softening of NiCu30Fe after annealing for 0.5 hrs and after 80% cold working]] | ||
| + | </figure> | ||
| + | </div> | ||
| + | <div class="clear"></div> | ||
| − | |||
| − | = | + | <figtable id="tab:Physical Properties of Nickel and Nickel Alloys"> |
| + | <caption>'''<!--Table 5.21:-->Physical Properties of Nickel and Nickel Alloys'''</caption> | ||
| − | + | {| class="twocolortable" style="text-align: left; font-size: 12px" | |
| + | |- | ||
| + | !Material<br />Designation<br />WST-Nr.<br />EN UNS | ||
| + | !Composition<br />[wt%] | ||
| + | !Density<br />[g/cm<sup>3</sup>] | ||
| + | !colspan="2" style="text-align:center"|Electrical<br />Conductivity | ||
| + | !Electrical<br />Resistivity<br />[μΩ·cm] | ||
| + | !Thermal<br />Conductivity<br />[W/(m·K)] | ||
| + | !Coeff. of Linear<br />Thermal<br />Expansion<br />[10<sup>-6</sup>/K] | ||
| + | !Modulus of<br />Elasticity<br />[GPa] | ||
| + | !Softening Temperature<br />(approx. 10% loss in<br />strength)<br />[°C] | ||
| + | !Melting<br />Temp Range<br />[°C] | ||
| + | |- | ||
| + | ! | ||
| + | ! | ||
| + | ! | ||
| + | ![MS/m] | ||
| + | ![% IACS] | ||
| + | ! | ||
| + | ! | ||
| + | ! | ||
| + | ! | ||
| + | ! | ||
| + | ! | ||
| + | |- | ||
| + | |Ni 99,2<br />2.4066<br />17740<br />N02200<br /> <br /> | ||
| + | |Mn < 0.35<br />Cu < 0.25<br />Si < 0.25<br />Fe < 0.4<br />C < 0.01<br />Ni > 99.2 | ||
| + | |8.9 | ||
| + | |11 | ||
| + | |19 | ||
| + | |9.0 | ||
| + | |70,5 | ||
| + | |13.0 | ||
| + | |207 | ||
| + | |ca. 450 | ||
| + | |1140 | ||
| + | |- | ||
| + | |NiCu30Fe<br />2.4360<br />17743<br />N04400 | ||
| + | |Cu 28 - 34<br />Fe 1 - 2.5<br />Ni Rest<br />Be 1.85 - 2.05 | ||
| + | |8.8 | ||
| + | |2.1 | ||
| + | |3.6 | ||
| + | |48.0 | ||
| + | |22 | ||
| + | |14.0 | ||
| + | |185 | ||
| + | |ca. 420 | ||
| + | |1300 - 1350 | ||
| + | |- | ||
| + | |NiBe2<br /> <br />N03360 | ||
| + | |Ti 0.4 - 0.6<br />Ni Rest | ||
| + | |8.3 | ||
| + | |5.0<sup>a</sup> | ||
| + | |8.6 | ||
| + | |0.2<sup>a</sup> | ||
| + | |48 | ||
| + | |14.4 | ||
| + | |210 | ||
| + | | | ||
| + | |1380 | ||
| + | |} | ||
| + | </figtable> | ||
| + | |||
| + | <sup>a</sup>solution annealed, and hardened | ||
| + | |||
| + | |||
| + | |||
| + | <figtable id="tab:Mechanical Properties of Nickel and Nickel Alloys"> | ||
| + | <caption>'''<!--Table 5.22:-->Mechanical Properties of Nickel and Nickel Alloys'''</caption> | ||
| + | |||
| + | {| class="twocolortable" style="text-align: left; font-size: 12px" | ||
| + | |- | ||
| + | !Material | ||
| + | !Hardness<br />Condition | ||
| + | !Tensile Strength R<sub>m</sub><br />[MPa] | ||
| + | !0,2% Yield Strength<br />R<sub>p02</sub><br />[MPa] | ||
| + | !Elongation<br />A<sub>50</sub><br />[%] | ||
| + | !Vickers<br />Hardness<br />HV | ||
| + | !Spring Bending<br />Limit σ<sub>FB</sub><br />[MPa] | ||
| + | !Fatigue<br />Strength σ<sub>BW</sub><br />[MPa] | ||
| + | |- | ||
| + | |Ni99,2 | ||
| + | |R 380 | ||
| + | |≥ 380 | ||
| + | |≥ 100 | ||
| + | |≥ 40 | ||
| + | |≥ 100 | ||
| + | | | ||
| + | | | ||
| + | |- | ||
| + | |NiCu30Fe | ||
| + | |R 400<br />R 700 | ||
| + | |400 - 600<br />700 - 850 | ||
| + | |≥ 160<br />≥ 600 | ||
| + | |≥ 30<br />≥ 4 | ||
| + | |95 - 125<br />200 - 240 | ||
| + | | | ||
| + | | | ||
| + | |- | ||
| + | |NiBe2 | ||
| + | |R 700<sup>a</sup><br />R 1300<sup>a</sup><br />R 1500<sup>b</sup><br />R 1900<sup>b</sup><br />R 1800<sup>c</sup> | ||
| + | |≥ 700<br />≥ 1300<br />≥ 1500<br />≥ 1900<br />≥ 1800 | ||
| + | |≥ 300<br />≥ 1200<br />≥ 1100<br />≥ 1750<br />≥ 1700 | ||
| + | |≥ 30<br />≥ 1<br />≥ 12<br />≥ 1<br />≥ 5 | ||
| + | |≥ 170<br />≥ 370<br />≥ 450<br />≥ 520<br />≥ 500 | ||
| + | | <br /> <br /> <br /> <br />≥ 1400 | ||
| + | |<br /> <br /> <br /> <br />≥ 400 | ||
| + | |} | ||
| + | </figtable> | ||
| − | + | <sup>a</sup>solution annealed, and cold rolled<br /> | |
| + | <sup>b</sup>solution annealed, cold rolled, and precipitation hardened<br /> | ||
| + | <sup>c</sup>solution annealed, cold rolled, and precipitation hardened at mill (mill hardened) | ||
| − | A further advantage of NiBe2 is its high temperature stability. Cold worked and subsequently precipitation hardened NiBe2 can withstand sustained | + | ===<!--5.2.3-->Nickel-Beryllium Alloys=== |
| + | |||
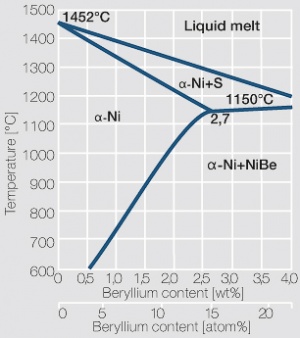
| + | Because of decreasing solubility of beryllium in nickel with decreasing temperature, NiBe can be precipitation hardened similar to CuBe (<xr id="fig:Phase diagram of nickel beryllium"/><!--(Fig. 5.49)-->). The maximum soluble amount of Be in Ni is 2.7 wt% at the eutectic temperature of 1150°C. To achieve a high hardness by precipitation hardening, NiBe similar to CuBe, is annealed at 970 - 1030°C and rapidly quenched to room temperature. Soft annealed material is easily cold formed and after stamping and forming a hardening anneal is performed at 480 to 500°C for 1 to 2 hours. | ||
| + | |||
| + | <figure id="fig:Phase diagram of nickel beryllium"> | ||
| + | [[File:Phase diagram of nickel beryllium.jpg|right|thumb|Figure 9: Phase diagram of nickel-beryllium]] | ||
| + | </figure> | ||
| + | |||
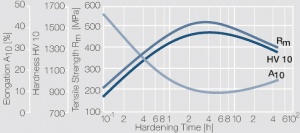
| + | Commercial nickel-beryllium alloys contain 2 wt% Be. Compared to CuBe2 the NiBe2 materials have a significantly higher modulus of elasticity but a much lower electrical conductivity. The mechanical strength is higher than that of CuBe2 (<xr id="fig:Precipitation hardening of NiBe2 soft at 480C"/><!--(Fig. 5.50)-->), the spring bending force limit can exceed values of over 1400 MPa and the fatigue strength reaches approximately 400 MPa. | ||
| + | |||
| + | <figure id="fig:Precipitation hardening of NiBe2 soft at 480C"> | ||
| + | [[File:Precipitation hardening of NiBe2 soft at 480C.jpg|right|thumb|Figure 10: Precipitation hardening of NiBe2 (soft) at 480°C]] | ||
| + | </figure> | ||
| + | |||
| + | A further advantage of NiBe2 is its high temperature stability. Cold worked and subsequently precipitation hardened, NiBe2 can withstand sustained | ||
temperatures of 400 - 650°C, depending on ist pre-treatment. | temperatures of 400 - 650°C, depending on ist pre-treatment. | ||
| − | Similar to CuBe materials, NiBe alloys are available in mill hardened | + | Similar to CuBe materials, NiBe alloys are available in various mill hardened conditions or also already precipitation hardened by the manufacturer. |
Nickel-beryllium alloys are recommended for mechanically and thermally highly stressed spring components. For some applications their ferro-magnetic properties can also be advantageous. | Nickel-beryllium alloys are recommended for mechanically and thermally highly stressed spring components. For some applications their ferro-magnetic properties can also be advantageous. | ||
| − | + | ==<!--5.3-->Triple-Layer Carrier Materials== | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Manufacturing of triple-layer carrier materials is usually performed by cold rollcladding. The three materials cover each other completely. The advantage of this composite material group is that the different mechanical and physical properties of the individual components can be combined with each other. | Manufacturing of triple-layer carrier materials is usually performed by cold rollcladding. The three materials cover each other completely. The advantage of this composite material group is that the different mechanical and physical properties of the individual components can be combined with each other. | ||
| − | Depending on the intended application the following layer systems are utilized: | + | Depending on the intended application, the following layer systems are utilized: |
* Conduflex N <br/> CuSn6 - Cu - CuSn6 <br/> | * Conduflex N <br/> CuSn6 - Cu - CuSn6 <br/> | ||
| Line 338: | Line 691: | ||
* Cu – Fe or Steel – Cu | * Cu – Fe or Steel – Cu | ||
| − | The high electrical conductivity and good arc mobility properties of copper are combined with the mechanical strength and magnetic properties of iron or steel. The thickness and width range of material strips are the same | + | The high electrical conductivity and good arc mobility properties of copper are combined with the mechanical strength and magnetic properties of iron or steel. The thickness and width range of material strips are the same as those for Cu – Invar – Cu system. |
The thickness ratios of the components can be selected according to the application requirements. The two outer layers usually have the same thickness. | The thickness ratios of the components can be selected according to the application requirements. The two outer layers usually have the same thickness. | ||
| − | ==5.4 Thermostatic Bimetals== | + | ==<!--5.4-->Thermostatic Bimetals== |
| − | Thermostatic bimetals are composite materials consisting of two or three layers of materials with different coefficients of thermal expansion. They are usually bonded together by cladding. If such a material part is heated either directly through current flow or indirectly through heat conduction or radiation, the different expansion between the active (strong expansion) and passive (low expansion) layer causes bending of the component part. | + | Thermostatic bimetals are composite materials, consisting of two or three layers of materials, with different coefficients of thermal expansion. They are usually bonded together by cladding. If such a material part is heated, either directly through current flow or indirectly through heat conduction or radiation, the different expansion between the active (strong expansion) and passive (low expansion) layer causes bending of the component part. |
| − | Directional or force effects on the free end of the thermostatic bimetal part is then used as a trigger or control mechanism in thermostats, protective switches | + | Directional or force effects on the free end of the thermostatic bimetal part is then used as a trigger or control mechanism in thermostats, protective switches or in control circuits. Depending on the required function of the thermostatic bimetal component different design shapes are used: |
*'''Straight or U-shaped strips''' for nearly linear motion | *'''Straight or U-shaped strips''' for nearly linear motion | ||
| Line 353: | Line 706: | ||
*'''Stamped and formed parts''' for special designs and applications | *'''Stamped and formed parts''' for special designs and applications | ||
| − | The wide variety of thermostatic bimetal types is specified mostly through DIN 1715 and/or applicable ASTM standards | + | The wide variety of thermostatic bimetal types is specified mostly through DIN 1715 and/or applicable ASTM standards (<xr id="tab:Partial Selection from the Wide Range of Available Thermo-Bimetals"/><!--(Table 5.23)-->). The different types have varying material compositions for the active and passive side of the materials. The mostly used alloys are iron-nickel and manganese-copper-nickel. Mainly used in circuit protection switches (i.e. circuit breakers), some thermo-bimetals include an intermediate layer of copper or nickel which allows to design parts with a closely controlled electrical resistance. |
| + | |||
| − | '''Table 5.23: Partial Selection from the Wide Range of Available Thermo-Bimetals''' | + | <figtable id="tab:Partial Selection from the Wide Range of Available Thermo-Bimetals"> |
| + | <caption>'''<!--Table 5.23:-->Partial Selection from the Wide Range of Available Thermo-Bimetals'''</caption> | ||
{| class="twocolortable" style="text-align: left; font-size: 12px" | {| class="twocolortable" style="text-align: left; font-size: 12px" | ||
| Line 391: | Line 746: | ||
|Three components with Ni intermediale layer | |Three components with Ni intermediale layer | ||
|} | |} | ||
| + | </figtable> | ||
| + | |||
| + | ===<!--5.4.1-->Design Formulas=== | ||
| + | |||
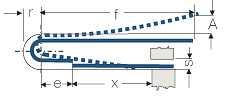
| + | For the design and calculation of the most important thermostatic-bimetal parts, formulas are given in (<xr id="tab:Design Formulas for Thermostatic Bimetal Components"/><!--Table 5.24-->). The necessary properties can be extracted for the most common materials from (<xr id="tab:Partial Selection from the Wide Range of Available Thermo-Bimetals"/><!--Table 5.23-->). The values given are valid only for a temperature range up to approximately 150°C. For higher temperatures; data can be obtained from the materials manufacturer. | ||
| + | |||
| + | |||
| + | <figtable id="tab:Design Formulas for Thermostatic Bimetal Components"> | ||
| + | <caption>'''<!--Table 5.24:-->Design Formulas for Thermostatic Bimetal Components'''</caption> | ||
| − | === | + | {| class="twocolortable" style="font-size:1em;" |
| + | |- | ||
| + | | | ||
| + | |Shape of the Thermostatic Bimetal | ||
| + | |Deflection | ||
| + | |Mechanical Action Force | ||
| + | |Thermal Action Force | ||
| + | |- | ||
| + | |Cantilevered strip | ||
| + | |[[File:Contilevered strip.jpg|left|234px|]] | ||
| + | |<math>A = | ||
| + | \frac {\alpha \Delta TL^2}{s} </math> | ||
| + | |<math>P = | ||
| + | \frac {cA Bs^3}{L^3} </math> | ||
| + | |<math>P = | ||
| + | \frac {b \Delta T Bs^3}{L} </math> | ||
| + | |- | ||
| + | |Dual supported strip | ||
| + | |[[File:Dual supported strip.jpg|left|234px|]] | ||
| + | |<math>A = | ||
| + | \frac {\alpha \Delta T L^2}{4s} </math> | ||
| + | |<math>P = | ||
| + | \frac {16c AB s^3}{L^3} </math> | ||
| + | |<math>P = | ||
| + | \frac {4b \Delta TB s^2}{L} </math> | ||
| + | |- | ||
| + | |U-shaped element | ||
| + | |[[File:U shaped element.jpg|left|220px|]] | ||
| + | |<math>A = | ||
| + | \frac {\alpha \Delta T L^2}{2s} </math> | ||
| + | |<math>P = | ||
| + | \frac {4c AB s^3}{L^3} </math> | ||
| + | |<math>P = | ||
| + | \frac {2b \Delta TB s^2}{L} </math> | ||
| + | |- | ||
| + | |Spiral | ||
| + | |[[File:Spiral.jpg|left|220px|]] | ||
| + | |colspan="3" style="text-align:center"|<math>A = | ||
| + | \frac {\alpha \Delta T}{s} (f^2 - e^2 + 4 r^2 + 2 e f + 2 \pi r f) </math> | ||
| + | |- | ||
| + | |Helical spring | ||
| + | |[[File:Helical spring.jpg|left|220px|]] | ||
| + | |<math>\alpha = | ||
| + | \frac {\alpha_{1} \Delta TL}{s} </math> | ||
| + | |<math>P = | ||
| + | \frac {c_{1} \alpha Bs^3}{L \cdot r} </math> | ||
| + | |<math>P = | ||
| + | \frac {b_{1} \Delta TBs^2}{r} </math> | ||
| + | |- | ||
| + | |Disc | ||
| + | |[[File:Disc.jpg|left|220px|]] | ||
| + | |<math>A = | ||
| + | \frac {\alpha \Delta T (D^2 - d^2)}{5s} </math> | ||
| + | |<math>P = | ||
| + | \frac {16c A s^3}{D^2 - d^2} </math> | ||
| + | |<math>P = 3,2 b \Delta T s^2 </math> | ||
| + | |- | ||
| + | |Reversed strip | ||
| + | |[[File:Reversed strip.jpg|left|240px|]] | ||
| + | |<math>A = | ||
| + | \frac {\alpha \Delta T}{s} (y^2 - 2xy - x^2) </math> | ||
| + | |<math>P = | ||
| + | \frac {c ABs^2}{L^3} </math> | ||
| + | |<math>P = | ||
| + | \frac {b \Delta T Bs^2}{L^3} (y^2 - 2xy - x^2) </math> | ||
| + | |- | ||
| + | |Reversed U-shaped element | ||
| + | |[[File:Reserved u shaped element.jpg|left|228px|]] | ||
| + | |colspan="3" style="text-align:center"|<math>A = | ||
| + | \frac {\alpha \Delta T}{s} [f^2 + 4 r^2 + 2 \pi r f - (e^2 - 2ex^2 - x^2) + 2f (e - x)] </math> | ||
| + | |} | ||
| + | </figtable> | ||
| − | + | {| style="border-spacing: 20px" | |
| + | |<math>A</math> || Deflection in mm | ||
| + | |<math>B</math> || Width in mm | ||
| + | | rowspan="2" |<math>a_{1} = \frac {360}{\pi} \cdot a</math> | ||
| + | |- | ||
| + | |<math>\alpha</math> || Turn angle in ° | ||
| + | |<math>D,d</math> || Diameter in mm | ||
| + | |- | ||
| + | |<math>P</math> || Force in N | ||
| + | |<math>r</math> || Radius in mm | ||
| + | | rowspan="2" |<math>b_{1} = \frac {2}{3} \cdot b</math> | ||
| + | |- | ||
| + | |<math>\Delta T</math> || Temperature difference in K | ||
| + | |<math>a</math> || Specific therm. Deflection in 1/K | ||
| + | |- | ||
| + | |<math>s</math> ||Thickness in mm | ||
| + | |<math>b=ac</math> ||Thermal action force constant<math> N/(mm^2 \cdot K)</math> | ||
| + | | rowspan="2" | <math>c_{1} = \frac {\pi}{540} \cdot c</math> | ||
| + | |- | ||
| + | |<math>L</math> || Free moving length in mm | ||
| + | |<math>c</math> || Mechan. action force constant in <math>N/mm^2</math> | ||
| + | |} | ||
| − | + | ===<!--5.4.2-->Stress Force Limitations=== | |
| − | = | + | For all calculations according to the formulas in (<xr id="tab:Design Formulas for Thermostatic Bimetal Components"/><!--Table 5.24-->) one should check if the thermally or mechanically induced stress forces stay below the allowed bending force limit. The following formulas are applicable for calculating the allowable load (Force P<sub>max</sub> or momentum M<sub>max</sub>): |
| − | |||
| − | + | <table class="twocolortable" style="text-align: left; font-size:12px;width:60%"> | |
| − | <math> | + | <tr> |
| − | + | <td>Single side fixed strip</td> | |
| − | {{ | + | <td><math>P_{max} < |
| − | + | \frac {\sigma Bs^2}{6L} </math> </td> | |
| − | + | </tr><tr> | |
| − | + | <td>Both sides fixed strip</td> | |
| − | + | <td><math>P_{max} < | |
| − | + | \frac {\sigma Bs^2}{1,5L} </math></td> | |
| − | + | </tr><tr> | |
| + | <td>Spiral or filament </td> | ||
| + | <td><math>M_{max} < | ||
| + | \frac {\sigma Bs^2}{6} </math></td> | ||
| + | </tr><tr> | ||
| + | <td>Disc </td> | ||
| + | <td><math>P_{max} < | ||
| + | \frac {2 \sigma s^2}{3} </math></td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <math>\sigma</math> = bending stress | ||
==Comments== | ==Comments== | ||
| Line 444: | Line 909: | ||
Kreye, H.; Nöcker, H.; Terlinde, G.: Schrumpfung und Verzug beim Aushärten von Kupfer-Beryllium-Legierungen. Metall 29 (1975) 1118-1121 | Kreye, H.; Nöcker, H.; Terlinde, G.: Schrumpfung und Verzug beim Aushärten von Kupfer-Beryllium-Legierungen. Metall 29 (1975) 1118-1121 | ||
| + | |||
| + | [[de:Trägerwerkstoffe]] | ||
Latest revision as of 09:49, 4 January 2023
The reliability and electrical life of contact systems in switching devices as well as in electromechanical and electronic components do not only depend on the contact material. The selection of the most suitable carrier material also plays an important role.
The most frequently used ones are copper based materials. Depending on the application, also materials based on nickel or multi-layer composite materials, such as thermo bimetals for example, are frequently used. For special applications in the medium and high voltage technology, as well as for springs and snap discs for the information technology, iron or steel based materials are considered. These are however not included for the purpose of this data book.
Various requirements based on the enduse of the contact components have to be met by carrier materials. Copper materials have to exhibit high electrical and thermal conductivity, good mechanical strength even at elevated temperatures and in addition, a sufficient high resistance against corrosion. If used as springs, the carrier materials also must have good elastic spring properties. Besides these, the materials must, depending on the manufacturing processes employed, also have good technological properties like ductility, to allow warm and cold forming, suitability for cutting and stamping and be capable to be welded, brazed or coated by electroplating.
Contents
Copper and Copper Alloys
Standards Overview
Copper and copper alloys being used in electrical and electronic components are usually covered by national and international standards. DIN numbers the materials by a prefix and/or a material number. The newer European standards (EN) refer to the material's usage products and also show a prefix and material number. For reference, we also show in (Table 1) the material designation according to UNS, the Unified Numbering System (USA). Other internationally used standard and material numbers include, among others, those issued by CDA (Copper Development Association, USA), and GB (Guo Biao – China).
The most important EN as well as the US based and widely used ASTM standards, covering the use of flat rolled copper and copper alloys in electrical contacts, are:
| Standard Designation | Description |
|---|---|
| DIN EN 1652 | Copper and copper alloys in plate, sheet, strip, and discs for general applications |
| DIN EN 1654 | Copper and copper alloys for springs and connectors |
| DIN EN 1758 | Copper and copper alloys in strip form for system component carriers |
| ASTM B 103/B103M-10 | Spec. for Phosphor Bronce Plate, Sheet, Strip, and Rolled Bar |
| ASTM B 36/B36M-95 | Spec. for Brass Plate, Sheet, Strip, and Rolled Bar |
| ASTM B 122/B122M-08 | Spec. for CuNiSn-, CuNiZn-, and CuNi-Alloy |
| ASTM B 465-09 | Spec. for Copper-Iron-Alloy Plate, Sheet, and Strip |
| ASTM B 194-08 | Standard Spec. for CuBe-Alloy Plate, Sheet, Strip and Rolled Bar |
| ASTM B 534-07 | Sec. for CuCoBe-Alloy and CuNiBe-Alloy Plate, Sheet, Strip, and Rolled Bar |
The above DIN EN standards replace in part or completely the older DIN standards DIN 1777, DIN 17670, DIN 1751, DIN 1791.
Pure Copper
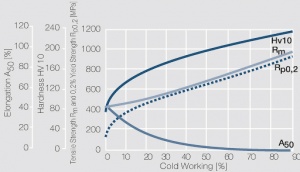
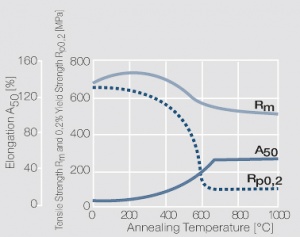
Copper is used in electrical engineering, mostly because of its high electrical conductivity[1] which with 58 MS/m (or m/Ωmm²) is only slightly below that of silver. Other advantages of copper are its high thermal conductivity, corrosion resistance and its good ductility. The work hardening properties of ETP copper is illustrated in (Figure 1). The increase in strength achieved by cold working can be reversed easily by subsequent annealing. The softening properties are strongly dependent on the preceding cold working percentage (Figure 2 and Figure 3).
The purity of technically pure and un-alloyed copper used for electrical applications depends on the type used and ranges between > 99.90 and 99.95 wt%. The copper types are designated mainly by their oxygen content as oxygen containing, oxygen-free, and de-oxidized with phosphorus as described in DIN EN 1652 (Table 1 and Table 2). Table 3 and Table 4 show the physical and mechanical properties of these copper materials. According to these, Cu-ETP, Cu-OFE and Cu-HCP are the types of copper for which minimum values for the electrical conductivity are guaranteed.
Cu-ETP is less suitable for welding or for brazing in reducing atmosphere because of the oxygen content (danger of hydrogen embrittlement).
Cu-HCP, Cu-DLP, and Cu-DHP are oxygen free copper types, de-oxidized with different phosphorus contents. With increasing phosphorus content, the electrical conductivity decreases. Cu-OFE, also called OFHC copper, is free of oxygen and also free of de-oxidizing compounds.
| WerkstMaterialEN-Designation | EN-Number | DIN-Designation | DIN-Number | UNS |
|---|---|---|---|---|
| Cu-ETP | CW004A | E-Cu 58 | 2.0065 | C11000 |
| Cu-OF | CW008A | OF-Cu | 2.0040 | C10200 |
| Cu-HCP | CW021A | SE-Cu | 2.0070 | C10300 |
| Cu-DLP | CW023A | SW-Cu | 2.0076 | C12000 |
| Cu-DHP | CW024A | F-Cu | 2.0090 | C12200 |
- Cu- ETP: electrolytic tough-pitch copper
- Cu-OFE: Oxygen Free Electronic Copper
- Cu-HCP: High Conductivity Phosphorus Deoxidized Copper
- Cu-DLP: phosphorous-deoxidized copper
- Cu-DHP: Phosphorous Deoxidized High Conductivity Copper
| Material | Composition (wt%) | |||||
|---|---|---|---|---|---|---|
| EN Designation | Cu | Bi | O | P | Pb | Others |
| Cu-ETP | >99.90 | bis 0.0005 | bis 0.040 | up to 0.005 | up to 0.03 | |
| Cu-OF | >99.95 | bis 0.0005 | up to 0.005 | up to 0.03 | ||
| Cu-HCP | >99.90 | ca. 0.003 | ||||
| Cu-DLP | >99.90 | bis 0.005 | 0.005-0.013 | up to 0.005 | up to 0.03 | |
| Cu-DHP | >99.90 | 0.015-0.040 | ||||
Material | Density | Electrical Conductivityt | Electrical Conductivity | Thermal Conductivity |
Coeff. of Linear Thermal Expansion |
Modulus of Elasticity | Softening Temperatur (approx. 10% loss in strength) | Melting Temperature | |
|---|---|---|---|---|---|---|---|---|---|
EN- Designation |
[g/cm³] | [MS/m] | [% IACS] | [μΩ· cm] | [W/(m· K)] | [10-6/K] | [GPa] | [°C] | [°C] |
Cu-ETP | 8.94 |
≥58 | 100 | 1.72 | 390 | 17.7 | 127 | ca. 220 | 1083 |
Cu-OF | 8.94 | ≥58 | 100 | 1.72 | 394 | 17.7 | 127 | ca. 220 | 1083 |
Cu-HCP | 8.94 | ≥54 | 93 | 1.85 | 380 | 17.7 | 127 | ca. 220 | 1083 |
Cu-DLP | 8.94 | 52 | 90 | 1.92 | 350 | 17.7 | 132 | ca. 220 | 1083 |
Cu-DHP | 8.94 | ≥46 | 80 | 2.17 | 310 | 17.6 | 132 | ca. 220 | 1083 |
Material | Condition | Tensile Strength Rm [MPa] | 0,2% Yield Strength Rp0,2 [MPa] | Elongation A50 [ %] | Hardness HV |
|---|---|---|---|---|---|
|
Cu-ETP Cu-OF Cu-HCP Cu-DLP Cu-DHP |
R220 | 220 - 260 | ≤140 | ≥33 | 40 - 65 |
| R240 | 240 - 300 | ≥180 | ≥8 | 65 - 95 | |
| R290 | 290 - 360 | ≥250 | ≥4 | 90 - 110 | |
| R360 | ≥360 | ≥320 | ≥2 | ≥110 |
High Cu Content Copper Alloys
The high Cu content alloy materials are closest in their properties to pure copper materials. By defined addition of small amounts of alloying elements, it is possible to increase the mechanical strength and especially the softening temperature of copper and at the same time decrease the electrical conductivity only insignificantly (Figure 4). Silver, iron, tin, zinc, nickel, chromium, zirconium, silicon and titanium are used. Usually, the additive amounts are significantly below 3 wt%. This group of materials consists of mixed crystal as well as precipitation hardening alloys. The precipitation hardening copper-beryllium and copper-chromium-zirconium materials are decribed later in a separate section.
From the large number of high-Cu alloys, only the properties of selected ones are covered here (Table 5 and Table 6). Some of these materials are not included in the EN standards system.
The low alloyed materials CuAg0.1 and CuCd1 are mostly used as overhead drive cables, where they have to meet sustained loads at elevated temperatures without softening.
The materials CuFe0.1 and CuSn0.15 have a high electrical conductivity. The mechanical strength of both is relatively low but stays almost constant at temperatures up to 400°C. They are used as substrates for power semiconductors and also as carriers for stationary contacts in higher energy switchgear.
CuFe2 is a material exhibiting high electrical conductivity and good formability. During an annealing process, Fe-rich precipitations are formed in the Cu matrix, which changes the mechanical properties very little but increases the electrical conductivity significantly. Besides being used as a contact carrier material in switching devices, this material has broader applications in automotive connectors and as a substrate in the semiconductor technology.
CuNi2Si has high mechanical strength, good formability and at the same time high electrical conductivity. This combination of advantageous properties is achieved by a defined finely dispersed precipitation of nickel silicides. CuNi2Si is used mainly in the form of stamped and formed parts in thermally stressed electromechanical components for automotive applications.
CuSn1CrNiTi and CuCrSiTi are advanced developments of the Cu-Cr-Ti precipitation materials with fine intermetallic dispersions. The material CuNi1Co1Si also belongs into this family and has properties similar to the low alloyed CuBe materials.
| Material/ Designation EN UNS |
Composition | Density [g/cm3] |
Electrical Conductivity |
Electrical Resistivity [μΩ·cm] |
Thermal Conductivity [W/(m·K)] |
Coeff. of Linear Thermal Expansion [10-6/K] |
Modulus of Elasticity [GPa] |
Softening Temperature (approx. 10% loss in strength) [°C] |
Melting Temp Range [°C] | |
|---|---|---|---|---|---|---|---|---|---|---|
| [MS/m] | [% IACS] | |||||||||
| CuAg 0,1 CW 013A |
Ag 0.08-0.12 Cu Rest |
8.89 | 56 | 97 | 1.8 | 380 | 17.7 | 126 | 1082 | |
| CuFe0,1P not standardized C19210 |
Fe 0.05-0.015 P 0.025-0.04 Cu Rest |
8.89 | 53 | 91 | 1.9 | 350 | 17.0 | 130 | ca. 280 | 1080 |
| CuSn0,15 CW117C C14415 |
Sn 0.1-0.15 Zn 0.1 Cu Rest |
8.93 | 51 | 88 | 2.0 | 350 | 18.0 | 130 | ca. 280 | 1060 |
| CuFe2P CW107C C19400 |
Fe 2.1-2.6 P 0.015-0.15 Zn 0.05-0.2 Cu Rest |
8.91 | 37 | 64 | 2.7 | 260 | 17.6 | 125 | ca. 380 | 1084 - 1090 |
| CuNi2Si CW111C C70260 |
Ni 1.6-2.5 Si 0.4-0.8 Fe 0.2 Cu Rest |
8.80 | 23 | 40 | 4.3 | 200 | 17.0 | 130 | ca. 430 | |
| CuSn1CrNiTi not standardized C18090 |
Sn 0.6 Ni 0.4 Cr 0.3 Ti 0.3 Cu Rest |
8.87 | 35 | 60 | 2.9 | 240 | 17.6 | 133 | ca. 480 | 1025 - 1074 |
| CuNi1Co1Si not standardized C70350 |
Ni 1.5 Co 1.1 Si 0.6 Cu Rest |
8.82 | 29 | 50 | 3.4 | 200 | 17.6 | 131 | ca. 400 | |
| CuCrSiTi not standardized C18070 |
Cr 0.3 Ti 0.1 Si 0.02 Cu Rest |
8.88 | 45 | 78 | 2.2 | 310 | 18.0 | 138 | ca. 430 | |
| Material | Hardness Condition |
Tensile Strength Rm [MPa] |
0,2% YieldStrength Rp02 [MPa] |
Elongation A50 [%] |
Vickers Hardness HV |
Bend Radius1) perpendicular to rolling direction |
Bend Radius1) parallel to rolling direction |
Spring Bending Limit σFB [MPa] |
Spring Fatigue Limit σBW [MPa] |
|---|---|---|---|---|---|---|---|---|---|
| CuAg0,10 | R 200 R 360 |
200 - 250 360 |
120 320 |
> 40 > 3 |
40 90 |
0 x t 0.5 x t |
0 x t 0.5 x t |
240 | 120 |
| CuFe0,1P | R 300 R 360 R 420 |
300 - 380 360 - 440 420 - 500 |
> 260 > 300 > 350 |
> 10 > 3 > 2 |
80 - 110 110 - 130 120 - 150 |
0 x t 0.5 x t 1.5 x t |
0 x t 0.5 x t 1.5 x t |
250 | 160 |
| CuSn0,15 | R 250 R 300 R 360 R 420 |
250 - 320 300 - 370 360 - 430 420 - 490 |
> 200 > 250 > 300 > 350 |
> 9 > 4 > 3 > 2 |
60 - 90 85 - 110 105 - 130 120 - 140 |
0 x t 0 x t 0 x t 1 x t |
0 x t 0 x t 0 x t 1 x t |
250 | 160 |
| CuFe2P | R 370 R 420 R 470 R 520 |
370 - 430 420 - 480 470 - 530 520 - 580 |
> 300 > 380 > 430 > 470 |
> 6 > 4 > 4 > 3 |
115 - 135 130 - 150 140 - 160 150 - 170 |
0 x t 0.5 x t 0.5 x t 1 x t |
0 x t 0.5 x t 0.5 x t 1 x t |
340 | 200 |
| CuNi2Si | R 430 R 510 R 600 |
430 - 520 510 - 600 600 - 680 |
> 350 > 450 > 550 |
> 10 > 7 > 5 |
125 - 155 150 - 180 180 - 210 |
0 x t 0 x t 1 x t |
0 x t 0 x t 1 x t |
500 | 230 |
| CuSn1CrNiTi | R 450 R 540 R 620 |
450 - 550 540 - 620 620 - 700 |
> 350 > 450 > 520 |
> 9 > 6 > 3 |
130 - 170 160 - 200 180 - 220 |
0.5 x t 1 x t 3 x t |
0.5 x t 2 x t 6 x t |
530 | 250 |
| CuNi1Co1Si | R 800 R 850 |
> 800 > 850 |
> 760 > 830 |
> 4 > 1 |
> 260 > 275 |
0.5 x t 1.5 x t |
1.5 x t 2.5 x t |
||
| CuCrSiTi | R 400 R 460 R 530 |
400 - 480 460 - 540 530 - 610 |
> 300 > 370 > 460 |
> 8 > 5 > 2 |
120 - 150 140 - 170 150 - 190 |
0 x t 0.5 x t 1 x t |
0 x t 0.5 x t 1 x t |
400 | 220 |
1) t: Strip thickness max. 0.5 mm
These newer copper based materials optimize properties, such as electrical conductivity, mechanical strength and relaxation, which are custom tailored to specific applications. Typical uses include contact springs for relays, switches and connectors.
Naturally Hard Copper Alloys
Alloys like brasses (CuZn), tin bronzes (CuSN) and German silver (CuNiZn), for which the required hardness is achieved by cold working, are defined as naturally hard alloys. Included in this group are also the silver bronzes (CuAg) with 2 – 6 wt% of Ag.
Main Articel: Naturally Hard Copper Alloys
Other Naturally Hard Copper Alloys
Main Articel: Other Naturally Hard Copper Alloys
Precipitation Hardening Copper Alloys
Besides the naturally hard copper materials, precipitation hardening copper alloys play also an important role as carrier materials for electrical contacts. By means of a suitable heat treatment, finely dispersed precipitations of a second phase can be achieved, which increases the mechanical strength of these copper alloys significantly.
Main Articel: Precipitation Hardening Copper Alloys
Application Properties for the Selection of Copper Alloys
Important for the usage as spring contact components are, besides mechanical strength and electrical conductivity, mainly the typical spring properties such as the maximum spring bending limit and the fatigue strength as well as the bendability. During severe thermal stressing the behavior of spring materials is determined by their softening and relaxation. The following briefly describes these material properties.
Main Articel: Application Properties for the Selection of Copper Alloys
Selection Criteria for Copper-Based Materials
The selection of copper-based materials from the broad spectrum of available materials must be based on the requirements of the application. First, an application profile should be established which can be used to define the material properties. Usually there is however no single material that can fulfill all requirements to the same degree. A compromise must be found as for example between electrical conductivity and spring properties.
If current carrying capability is the key requirement, mechanical strength may have to be sacrificed as for example in carrier parts for stationary contacts. In this case, depending on the current level, pure copper or low alloyed copper materials such as CuSn0.15, or for economic reasons CuZn30, may be suitable.
For spring contact components, the interdependent relations between electrical conductivity and fatigue strength, or electrical conductivity and relaxation behavior are of main importance. The first case is critical for higher load relay springs. CuAg2 plays an important role for these applications. The latter is critical for components that are exposed to continuing high mechanical stresses like for example in connectors. The spring force must stay close to constant over the expected life time of the parts, even at elevated temperatures from the environment or current carrying. In this case, the relaxation behavior of the copper materials, which may cause a decrease in spring force over time, must be considered. Besides this, easy forming during manufacturing must be possible; this means that bending operations can also be performed at high mechanical strength values.
The increasing requirements on spring components in connectors, especially for use in automotive applications, such as higher surrounding temperatures, increased reliability and the trend towards miniaturization led to a change of materials from traditionally CuZn30 and CuSn4 to CuNiSi alloys, for example. These CuNiSi alloys and the newer heavy duty copper alloys like CuNi1Co1, are significantly improved with regards to mechanical strength, relaxation behavior and electrical conductivity.
Nickel and Nickel Alloys
Technical Grade Pure Nickel
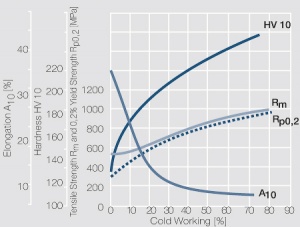
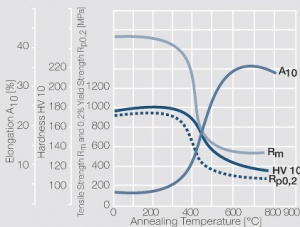
Technical grade pure nickel commonly contains 99.0 to 99.8 wt% Ni and up to 1 wt% Co. Other ingredients are iron and manganese (Table 7 and Table 8). Work hardening and softening behavior of nickel are shown in (Figs. 5 – 6).
One of the significant properties of nickel is its modulus of elasticity, which is almost twice as high as that of copper. At temperatures up to 345°C nickel is ferro-magnetic. Nickel has a high corrosion resistance, is very ductile and easy to weld and clad. It is of great importance as a backing material for multiple layer weld profiles. In addition, nickel is used as an intermediate layer for thin claddings, acting as an effective diffusion barrier between copper containing carrier materials and gold or palladium-based contact materials.
Because of the always present thin oxide layer on its surface, nickel is not suitable as a contact material for switching contacts.
Nickel Alloys
Because of its low electrical conductivity, NiCu30Fe is besides pure Ni and CuNi alloys the most widely used backing material for weldable contact components. With 1 – 2 wt% additives of Fe as well as 0.5 – 1 wt% Mn and Co, the mechanical strength of the binary alloy NiCu30 can be increased.
The strength values of NiCu30Fe are significantly higher than those of the copper rich CuNi alloys (Figs. 7 – 8). The good spring properties and thermal stability of NiCu30Fe make it a suitable material for the use as thermally stressed contact springs.
| Material Designation WST-Nr. EN UNS |
Composition [wt%] |
Density [g/cm3] |
Electrical Conductivity |
Electrical Resistivity [μΩ·cm] |
Thermal Conductivity [W/(m·K)] |
Coeff. of Linear Thermal Expansion [10-6/K] |
Modulus of Elasticity [GPa] |
Softening Temperature (approx. 10% loss in strength) [°C] |
Melting Temp Range [°C] | |
|---|---|---|---|---|---|---|---|---|---|---|
| [MS/m] | [% IACS] | |||||||||
| Ni 99,2 2.4066 17740 N02200 |
Mn < 0.35 Cu < 0.25 Si < 0.25 Fe < 0.4 C < 0.01 Ni > 99.2 |
8.9 | 11 | 19 | 9.0 | 70,5 | 13.0 | 207 | ca. 450 | 1140 |
| NiCu30Fe 2.4360 17743 N04400 |
Cu 28 - 34 Fe 1 - 2.5 Ni Rest Be 1.85 - 2.05 |
8.8 | 2.1 | 3.6 | 48.0 | 22 | 14.0 | 185 | ca. 420 | 1300 - 1350 |
| NiBe2 N03360 |
Ti 0.4 - 0.6 Ni Rest |
8.3 | 5.0a | 8.6 | 0.2a | 48 | 14.4 | 210 | 1380 | |
asolution annealed, and hardened
| Material | Hardness Condition |
Tensile Strength Rm [MPa] |
0,2% Yield Strength Rp02 [MPa] |
Elongation A50 [%] |
Vickers Hardness HV |
Spring Bending Limit σFB [MPa] |
Fatigue Strength σBW [MPa] |
|---|---|---|---|---|---|---|---|
| Ni99,2 | R 380 | ≥ 380 | ≥ 100 | ≥ 40 | ≥ 100 | ||
| NiCu30Fe | R 400 R 700 |
400 - 600 700 - 850 |
≥ 160 ≥ 600 |
≥ 30 ≥ 4 |
95 - 125 200 - 240 |
||
| NiBe2 | R 700a R 1300a R 1500b R 1900b R 1800c |
≥ 700 ≥ 1300 ≥ 1500 ≥ 1900 ≥ 1800 |
≥ 300 ≥ 1200 ≥ 1100 ≥ 1750 ≥ 1700 |
≥ 30 ≥ 1 ≥ 12 ≥ 1 ≥ 5 |
≥ 170 ≥ 370 ≥ 450 ≥ 520 ≥ 500 |
≥ 1400 |
≥ 400 |
asolution annealed, and cold rolled
bsolution annealed, cold rolled, and precipitation hardened
csolution annealed, cold rolled, and precipitation hardened at mill (mill hardened)
Nickel-Beryllium Alloys
Because of decreasing solubility of beryllium in nickel with decreasing temperature, NiBe can be precipitation hardened similar to CuBe (Figure 9). The maximum soluble amount of Be in Ni is 2.7 wt% at the eutectic temperature of 1150°C. To achieve a high hardness by precipitation hardening, NiBe similar to CuBe, is annealed at 970 - 1030°C and rapidly quenched to room temperature. Soft annealed material is easily cold formed and after stamping and forming a hardening anneal is performed at 480 to 500°C for 1 to 2 hours.
Commercial nickel-beryllium alloys contain 2 wt% Be. Compared to CuBe2 the NiBe2 materials have a significantly higher modulus of elasticity but a much lower electrical conductivity. The mechanical strength is higher than that of CuBe2 (Figure 10), the spring bending force limit can exceed values of over 1400 MPa and the fatigue strength reaches approximately 400 MPa.
A further advantage of NiBe2 is its high temperature stability. Cold worked and subsequently precipitation hardened, NiBe2 can withstand sustained temperatures of 400 - 650°C, depending on ist pre-treatment.
Similar to CuBe materials, NiBe alloys are available in various mill hardened conditions or also already precipitation hardened by the manufacturer.
Nickel-beryllium alloys are recommended for mechanically and thermally highly stressed spring components. For some applications their ferro-magnetic properties can also be advantageous.
Triple-Layer Carrier Materials
Manufacturing of triple-layer carrier materials is usually performed by cold rollcladding. The three materials cover each other completely. The advantage of this composite material group is that the different mechanical and physical properties of the individual components can be combined with each other.
Depending on the intended application, the following layer systems are utilized:
- Conduflex N
CuSn6 - Cu - CuSn6
The high electrical and thermal conductivity as well as the current carrying capacity of copper is combined with the spring properties of the tin bronze. Conduflex N strips are used in a thickness range of 0.1 – 1,5 mm in a maximum width of 140 mm.
- Cu - FeNi36 (Invar) - Cu
The high electrical conductivity and ductility of copperis combined with the low coefficient of thermal conductivity of the Invar alloy. The dimensionsional range is 0.2 – 1.8 mm in thickness with a maximum width of 140 mm.
- Cu – Fe or Steel – Cu
The high electrical conductivity and good arc mobility properties of copper are combined with the mechanical strength and magnetic properties of iron or steel. The thickness and width range of material strips are the same as those for Cu – Invar – Cu system.
The thickness ratios of the components can be selected according to the application requirements. The two outer layers usually have the same thickness.
Thermostatic Bimetals
Thermostatic bimetals are composite materials, consisting of two or three layers of materials, with different coefficients of thermal expansion. They are usually bonded together by cladding. If such a material part is heated, either directly through current flow or indirectly through heat conduction or radiation, the different expansion between the active (strong expansion) and passive (low expansion) layer causes bending of the component part.
Directional or force effects on the free end of the thermostatic bimetal part is then used as a trigger or control mechanism in thermostats, protective switches or in control circuits. Depending on the required function of the thermostatic bimetal component different design shapes are used:
- Straight or U-shaped strips for nearly linear motion
- Circular discs for small linear motions with high force
- Spirals and filament spring shapes for circular motion
- Stamped and formed parts for special designs and applications
The wide variety of thermostatic bimetal types is specified mostly through DIN 1715 and/or applicable ASTM standards (Table 9). The different types have varying material compositions for the active and passive side of the materials. The mostly used alloys are iron-nickel and manganese-copper-nickel. Mainly used in circuit protection switches (i.e. circuit breakers), some thermo-bimetals include an intermediate layer of copper or nickel which allows to design parts with a closely controlled electrical resistance.
| Designation DIN 1715 |
Designation ASTM |
Specific Thermal Deflection [106/K] |
Sprecific Electrical Resistance k [μΩ·m] |
Typical Application Range [°C] |
Application Limit [°C] |
Composition |
|---|---|---|---|---|---|---|
| TB 20110 TB 1577A TB1170A |
TM 2 TM 8 TM 1 TM 3 TM 4 |
21.1 15.3 15.5 14.2 11.7 10.6 8.5 |
1.12 1.41 0.79 0.78 0.70 0.71 0.66 |
- 70 – + 260 - 70 – + 260 - 70 – + 370 - 70 – + 370 - 70 – + 425 - 70 – + 480 - 70 – + 425 |
350 350 450 450 480 540 540 |
Two components |
| TB 1517 TB 1511 TB 1303 TB 1109 |
TM 28 TM 26 TM 25 TM 24 |
14.9 14.9 14.3 13.9 13.2 13.1 12.3 11.5 |
0.17 0.11 0.15 0.08 0.03 0.05 0.03 0.09 |
- 70 – + 260 - 70 – + 260 - 70 – + 315 - 70 – + 315 - 70 – + 260 - 70 – + 315 - 70 – + 315 - 70 – + 380 |
400 400 350 350 300 350 350 400 |
Three components with Cu intermediale layer |
| TB 1555 TB 1435 TB 1425 |
TM 17 TM 15 TM 13 TM 11 TM 9 |
15.0 14.8 14.2 14.1 14.0 13.6 12.8 10.7 |
0.55 0.40 0.66 0.50 0.25 0.33 0.25 0.17 |
- 70 – + 260 - 70 – + 260 - 70 – + 370 - 70 – + 370 - 70 – + 260 - 70 – + 370 - 70 – + 370 - 70 – + 370 |
450 450 480 480 450 480 480 480 |
Three components with Ni intermediale layer |
Design Formulas
For the design and calculation of the most important thermostatic-bimetal parts, formulas are given in (Table 10). The necessary properties can be extracted for the most common materials from (Table 9). The values given are valid only for a temperature range up to approximately 150°C. For higher temperatures; data can be obtained from the materials manufacturer.
| Shape of the Thermostatic Bimetal | Deflection | Mechanical Action Force | Thermal Action Force | |
| Cantilevered strip | ||||
| Dual supported strip | ||||
| U-shaped element | ||||
| Spiral | ||||
| Helical spring | ||||
| Disc | ||||
| Reversed strip | ||||
| Reversed U-shaped element | ||||
| Deflection in mm | Width in mm | |||
| Turn angle in ° | Diameter in mm | |||
| Force in N | Radius in mm | |||
| Temperature difference in K | Specific therm. Deflection in 1/K | |||
| Thickness in mm | Thermal action force constant |
|||
| Free moving length in mm | Mechan. action force constant in |
Stress Force Limitations
For all calculations according to the formulas in (Table 10) one should check if the thermally or mechanically induced stress forces stay below the allowed bending force limit. The following formulas are applicable for calculating the allowable load (Force Pmax or momentum Mmax):
| Single side fixed strip | |
| Both sides fixed strip | |
| Spiral or filament | |
| Disc |
= bending stress
Comments
- ↑ As units for electrical conductivity MS/m and m/Ω.mm2 are commonly used. Frequently – and mostly in North America – the % IACS value (International Annealed Copper Standard) is also used, where 100% is equivalent to 58 MS/m or m/Ωmm2 .For the description of mechanical strength properties the units of N/mm2 or MPa are most commonly used: 1 MS/m = 1 m/Ωmm2 1 MPa = 1 N/mm2
References
ASM Handbuch Volume 2, 10th Edition: Properties and Selection of Nonferrous
Alloys and Special Purpose Materials, ASM International, Cleveland OH, USA 1990
Wieland-Kupferwerkstoffe. Wieland-Werke AG, Ulm 1999
Rau, G.: Metallische Verbundwerkstoffe. Werkstofftechnische
Verlagsgesellschaft, Karlsruhe 1977
Kayser, O., Pawlek, F., Reichel, K.: Die Beeinflussung der Leitfähigkeit reinsten
Kupfers durch Beimengungen. Metall 8 (1954) 532-537
Dies, K.: Kupfer und Kupferlegierungen in der Technik. Springer-Verlag, Berlin, Heidelberg, New York, 1967
Gerlach,U.; Kreye, H.: Gefüge und mechanische Eigenschaften der Legierung
CuNi9Sn2. Metall 32 (1978) 1112-1115
Beryvac, Firmenschrift Vakuumschmelze GmbH, Hanau 1974
Beryvac 520, Firmenschrift Vacuumschmelze GmbH, Hanau 1975
Kupfer-Beryllium, Firmenschrift Brush Wellman
Kreye, H.; Nöcker, H.; Terlinde, G.: Schrumpfung und Verzug beim Aushärten von Kupfer-Beryllium-Legierungen. Metall 29 (1975) 1118-1121