|
|
| (75 intermediate revisions by 6 users not shown) |
| Line 1: |
Line 1: |
| − | ===2.1 Introduction===
| + | The contact parts are important components in switching devices. They have to maintain their function from the new state until the end of the functional life of the devices. |
| − | The contact parts are important components in switching devices. They have to | |
| − | maintain their function from the new state until the end of the functional life of the | |
| − | devices. | |
| | | | |
| − | The requirements on contacts are rather broad. Besides typical contact properties | + | The requirements on contacts are rather broad. Besides typical contact properties such as |
| − | such as | |
| | | | |
| | *High arc erosion resistance | | *High arc erosion resistance |
| Line 13: |
Line 9: |
| | *Good arc extinguishing capability | | *Good arc extinguishing capability |
| | | | |
| − | they have to exhibit physical, mechanical, and chemical properties like high electrical
| + | They have to exhibit physical, mechanical and chemical properties like high electrical and thermal conductivity, high hardness, high corrosion resistance etc. and besides this, should have good mechanical workability and also be suitable for good weld and brazing attachment to contact carriers. In addition they must be made from environmentally friendly materials. |
| − | and thermal conductivity, high hardness, high corrosion resistance, etc and besides | |
| − | this should have good mechanical workability, and also be suitable for good weld and | |
| − | brazing attachment to contact carriers. In addition they must be made from | |
| − | environmentally friendly materials. | |
| | | | |
| − | Materials suited for use as electrical contacts can be divided into the following groups | + | Materials suited for use as electrical contacts can be divided into the following groups based on their composition and metallurgical structure: |
| − | based on their composition and metallurgical structure: | |
| | | | |
| | *Pure metals | | *Pure metals |
| | *Alloys | | *Alloys |
| | *Composite materials | | *Composite materials |
| − | *Pure metals
| |
| | | | |
| − | From this group silver has the greatest importance for switching devices in the higher
| |
| − | energy technology. Other precious metals such as gold and platinum are only used in
| |
| − | applications for the information technology in the form of thin surface layers. As a nonprecious
| |
| − | metal tungsten is used for some special applications such as for example as
| |
| − | automotive horn contacts. In some rarer cases pure copper is used but mainly paired
| |
| − | to a silver-based contact material.
| |
| | | | |
| − | *Alloys
| + | '''Pure metals''' |
| | + | |
| | + | Within this group, silver has the greatest importance for switching devices in the higher energy technology. Other precious metals such as gold and platinum are only used in applications for the information technology in the form of thin surface layers. As a nonprecious metal, tungsten is used for some special applications such as, for example, automotive horn contacts. In some rarer cases, pure copper is used, but mainly paired to a silver-based contact material. |
| | + | |
| | + | '''Alloys''' |
| | + | |
| | + | Besides these few pure metals, a larger number of alloy materials made by melt technology are available for the use as contacts. An alloy is characterized by the fact, that its components are completely or partially soluble in each other in the solid state. Phase diagrams for multiple metal compositions show the number and type of the crystal structure as a function of the temperature and composition of the alloying components. |
| | | | |
| − | Besides these few pure metals a larger number of alloy materials made by melt
| + | They indicate the boundaries of liquid and solid phases and define the parameters of solidification. |
| − | technology are available for the use as contacts. An alloy is characterized by the fact
| + | Alloying allows to improve the properties of one material at the cost of changing them for the second material. As an example, the hardness of a base metal may be increased while at the same time the electrical conductivity decreases with even small additions of the second alloying component. |
| − | that its components are completely or partially soluble in each other in the solid state.
| |
| − | Phase diagrams for multiple metal compositions show the number and type of the
| |
| − | crystal structure as a function of the temperature and composition of the alloying components.
| |
| | | | |
| − | They indicate the boundaries of liquid and solid phases and define the
| + | '''Composite Materials''' |
| − | parameters of solidification.
| |
| − | Alloying allows to improve the properties of one material at the cost of changing
| |
| − | them for the second material. As an example, the hardness of a base metal may
| |
| − | be increased while at the same time the electrical conductivity decreases with
| |
| − | even small additions of the second alloying component.
| |
| | | | |
| − | *Composite Materials
| + | Composite materials are a material group whose properties are of great importance for electrical contacts that are used in switching devices for higher |
| | + | electrical currents. |
| | | | |
| − | Composite materials are a material group whose properties are of great
| + | Those used in electrical contacts are heterogeneous materials, composed of two or more uniformly dispersed components, in which the largest volume portion consists of a metal. |
| − | importance for electrical contacts that are used in switching devices for higher
| |
| − | electrical currents.
| |
| − | Those used in electrical contacts are heterogeneous materials composed of two | |
| − | or more uniformly dispersed components in which the largest volume portion | |
| − | consists of a metal. | |
| − | The properties of composite materials are determined mainly independent from
| |
| − | each other by the properties of their individual components. Therefore it is for
| |
| − | example possible to combine the high melting point and arc erosion resistance
| |
| − | of tungsten with the low melting and good electrical conductivity of copper, or
| |
| − | the high conductivity of silver with the weld resistant metalloid graphite.
| |
| | | | |
| − | Figure 2.1 shows the schematic manufacturing processes from powder
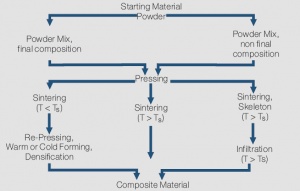
| + | The properties of composite materials are determined mainly independent from each other by the properties of their individual components. Therefore it is, for example, possible to combine the high melting point and arc erosion resistance of tungsten with the low melting and good electrical conductivity of copper or the high conductivity of silver with the weld resistant metalloid graphite. <xr id="fig:Powder metallurgical manufacturing of composite materials (schematic)"/> shows the schematic manufacturing processes from powder blending to contact material. Three basic process variations are typically applied: |
| − | blending to contact material. Three basic process variations are typically | |
| − | applied: | |
| | | | |
| | *Sintering without liquid phase (Press-Sinter-Repress, PSR) | | *Sintering without liquid phase (Press-Sinter-Repress, PSR) |
| Line 71: |
Line 42: |
| | *Infiltration (Press-Sinter-Infiltrate, PSI) | | *Infiltration (Press-Sinter-Infiltrate, PSI) |
| | | | |
| − | During sintering without a liquid phase (left side of schematic) the powder mix is
| + | <figure id="fig:Powder metallurgical manufacturing of composite materials (schematic)"> |
| − | first densified by pressing, then undergoes a heat treatment (sintering), and
| + | [[File:Powder metallurgical manufacturing of composite materials (schematic).jpg|thumb|<caption>Powder-metallurgical manufacturing of composite materials (schematic) T<sub>s</sub> = Melting point of the lower melting component)</caption>]] |
| − | eventually is re-pressed again to further increase the density. The sintering
| + | </figure> |
| − | atmosphere depends on the material components and later application; a
| |
| − | vacuum is used for example for the low gas content material Cu/Cr. This
| |
| − | process is used for individual contact parts and also termed press-sinterrepress
| |
| − | (PSR). For materials with high silver content the starting point at | |
| − | pressing is most a larger block (or billet) which is then after sintering hot
| |
| − | extruded into wire, rod, or strip form. The extrusion further increases the density
| |
| − | of these composite materials and contributes to higher arc erosion resistance.
| |
| − | Materials such as Ag/Ni, Ag/MeO, and Ag/C are typically produced by this
| |
| − | process.
| |
| − | | |
| − | Sintering with liquid phase has the advantage of shorter process times due to
| |
| − | the accelerated diffusion and also results in near-theoretical densities of the
| |
| | | | |
| − | Fig. 2.1: Powder-metallurgical manufacturing of composite materials (schematic)
| + | During ''sintering without a liquid phase'' (left side of schematic), the powder mix is first densified by pressing, then undergoes a heat treatment (sintering) and eventually is re-pressed again to further increase the density. The sintering atmosphere depends on the material components and later application; a vacuum is used for example for the low gas content material Cu/Cr. This process is used for individual contact parts and also termed press-sinter-repress (PSR). For materials with high silver content, the starting point before pressing is mostly a large block (or billet) which is then, after sintering, hot extruded into wire, rod or strip form. The extrusion further increases the density of these composite materials and contributes to higher arc erosion resistance. Materials such as Ag/Ni, Ag/MeO and Ag/C are typically produced by this process. |
| − | T = Melting point of the lower melting component
| |
| | | | |
| − | composite material. To ensure the shape stability during the sintering process it | + | ''Sintering with liquid phase'' has the advantage of shorter process times due to the accelerated diffusion and also results in near-theoretical densities of the composite material. To ensure the shape stability during the sintering process, it |
| | is however necessary to limit the volume content of the liquid phase material. | | is however necessary to limit the volume content of the liquid phase material. |
| | | | |
| − | As opposed to the liquid phase sintering which has limited use for electrical | + | As opposed to the liquid phase sintering, which has limited use for electrical contact manufacturing, the ''Infiltration process'' as shown on the right side of the schematic, has a broad practical range of applications. In this process the powder of the higher melting component, sometimes also as a powder mix with a small amount of the second material, is pressed into parts. Then, right after sintering, the porous skeleton is infiltrated with liquid metal of the second material. The fill-up process of the pores happens through capillary forces. This process reaches, after the infiltration, near-theoretical density without subsequent pressing and is widely used for Ag- and Cu-refractory contacts. For Ag/W or Ag/WC contacts, controlling the amount or excess on the bottom side of the contact of the infiltration metal Ag, results in contact tips that can be easily attached to their carriers by resistance welding. For larger Cu/W contacts, additional machining is often used to obtain the final shape of the contact component. |
| − | contact manufacturing, the Infiltration process as shown on the right side of the | |
| − | schematic has a broad practical range of applications. In this process the | |
| − | powder of the higher melting component sometimes also as a powder mix with | |
| − | a small amount of the second material is pressed into parts and after sintering | |
| − | the porous skeleton is infiltrated with liquid metal of the second material. The | |
| − | filling up of the pores happens through capillary forces. This process reaches
| |
| − | after the infiltration near-theoretical density without subsequent pressing and is | |
| − | widely used for Ag- and Cu-refractory contacts. For Ag/W or Ag/WC contacts, | |
| − | controlling the amount or excess on the bottom side of the contact of the | |
| − | infiltration metal Ag results in contact tips that can be easily attached to their | |
| − | carriers by resistance welding. For larger Cu/W contacts additional machining is | |
| − | often used to obtain the final shape of the contact component. | |
| − | | |
| − | ===2.2 Gold Based Materials===
| |
| − | | |
| − | Pure Gold is besides Platinum the chemically most stable of all precious metals.
| |
| − | In its pure form it is not very suitable for use as a contact material in
| |
| − | electromechanical devices because of its tendency to stick and cold-weld at even
| |
| − | low contact forces. In addition it is not hard or strong enough to resist
| |
| − | mechanical wear and exhibits high materials losses under electrical arcing
| |
| − | loads. This limits its use in form of thin electroplated or vacuum deposited layers.
| |
| − | | |
| − | For most electrical contact applications gold alloys are used. Depending on the
| |
| − | alloying metal the melting is performed either under in a reducing atmosphere or
| |
| − | in a vacuum. The choice of alloying metals depends on the intended use of the
| |
| − | resulting contact material. The binary Au alloys with typically <10 wt% of other
| |
| − | precious metals such as Pt, Pd, or Ag or non-precious metals like Ni, Co, and
| |
| − | Cu are the more commonly used ones (Table 2.2). On one hand these alloy
| |
| − | additions improve the mechanical strength and electrical switching properties
| |
| − | but on the other hand reduce the electrical conductivity and chemical corrosion
| |
| − | resistance (Fig. 2.2) to varying degrees.
| |
| − | | |
| − | Under the aspect of reducing the gold content ternary alloys with a gold content
| |
| − | of approximately 70 wt% and additions of Ag and Cu or Ag and Ni resp., for
| |
| − | example AuAg25Cu5 or AuAg20Cu10 are used which exhibit for many
| |
| − | applications good mechanical stability while at the same time have sufficient
| |
| − | resistance against the formation of corrosion layers (Table 2.3). Other ternary
| |
| − | alloys based on the AuAg system are AuAg26Ni3 and AuAg25Pt6. These alloys
| |
| − | are mechanically similar to the AuAgCu alloys but have significantly higher
| |
| − | oxidation resistance at elevated temperatures (Table 2.4).
| |
| − | | |
| − | Caused by higher gold prices over the past years the development of alloys with
| |
| − | further reduced gold content had a high priority. The starting point has been the
| |
| − | AuPd system which has continuous solubility of the two components. Besides
| |
| − | the binary alloy of AuPd40 and the ternary one AuPd35Ag9 other multiple
| |
| − | component alloys were developed. These alloys typically have < 50 wt% Au and
| |
| − | often can be solution hardened in order to obtain even higher hardness and
| |
| − | tensile strength. They are mostly used in sliding contact applications.
| |
| − | | |
| − | Gold alloys are used in the form of welded wire or profile (also called weldtapes),
| |
| − | segments, contact rivets, and stampings produced from clad strip
| |
| − | materials. The selection of the bonding process is based on the cost for the
| |
| − | joining process, and most importantly on the economical aspect of using the
| |
| − | least possible amount of the expensive precious metal component.
| |
| − | | |
| − | Besides being used as switching contacts in relays and pushbuttons, gold
| |
| − | alloys are also applied in the design of connectors as well as sliding contacts for
| |
| − | potentiometers, sensors, slip rings, and brushes in miniature DC motors
| |
| − | (Table 2.5).
| |
| − | | |
| − | Table 2.3: Mechanical Properties of Gold and Gold-Alloys
| |
| − | | |
| − | Table 2.1: Commonly Used Grades of Gold
| |
| − | | |
| − | Table 2.2: Physical Properties of Gold and Gold-Alloys
| |
| − | | |
| − | Fig. 2.2:
| |
| − | Influence of 1-10 atomic% of different
| |
| − | alloying metals on the electrical resistivity of gold
| |
| − | (according to J. O. Linde)
| |
| − | | |
| − | Fig. 2.3:
| |
| − | Phase diagram
| |
| − | of goldplatinum
| |
| − | | |
| − | Fig. 2.4:
| |
| − | Phase diagram
| |
| − | of gold-silver
| |
| − | | |
| − | Fig. 2.5:
| |
| − | Phase diagram
| |
| − | of gold-copper
| |
| − | | |
| − | Fig. 2.6: Phase diagram of gold-nickel
| |
| − | | |
| − | Fig. 2.7: Phase diagram of gold-cobalt
| |
| − | | |
| − | Fig. 2.8:
| |
| − | Strain hardening
| |
| − | of Au by cold working
| |
| − | | |
| − | Fig. 2.9:
| |
| − | Softening of Au after annealing
| |
| − | for 0.5 hrs after 80%
| |
| − | cold working
| |
| − | | |
| − | Fig. 2.10:
| |
| − | Strain hardening of
| |
| − | AuPt10 by cold working
| |
| − | | |
| − | Fig. 2.11:
| |
| − | Strain hardening
| |
| − | of AuAg20 by cold working
| |
| − | | |
| − | Fig. 2.12:
| |
| − | Strain hardening of
| |
| − | AuAg30 by cold working
| |
| − | | |
| − | Fig. 2.13:
| |
| − | Strain hardening of AuNi5
| |
| − | by cold working
| |
| − | | |
| − | Fig. 2.14:
| |
| − | Softening
| |
| − | of AuNi5 after annealing
| |
| − | for 0.5 hrs after 80%
| |
| − | cold working
| |
| − | | |
| − | Fig. 2.15:
| |
| − | Strain hardening
| |
| − | of AuCo5 by cold working
| |
| − | | |
| − | Fig. 2.16:
| |
| − | Precipitation hardening of
| |
| − | AuCo5 at 400°C hardening
| |
| − | temperature
| |
| − | | |
| − | Fig. 2.17:
| |
| − | Strain hardening
| |
| − | of AuAg25Pt6 by cold working
| |
| − | | |
| − | Fig. 2.18:
| |
| − | Strain hardening
| |
| − | of AuAg26Ni3 by cold working
| |
| − | | |
| − | Fig. 2.19:
| |
| − | Softening
| |
| − | of AuAg26Ni3 after
| |
| − | annealing for 0.5 hrs
| |
| − | after 80% cold
| |
| − | working
| |
| − | | |
| − | Fig. 2.20:
| |
| − | Strain hardening of
| |
| − | AuAg25Cu5
| |
| − | by cold working
| |
| − | | |
| − | Fig. 2.21:
| |
| − | Strain hardening of
| |
| − | AuAg20Cu10
| |
| − | by cold working
| |
| − | | |
| − | Fig. 2.22:
| |
| − | Softening
| |
| − | of AuAg20Cu10 after
| |
| − | annealing for 0.5 hrs
| |
| − | after 80% cold working
| |
| − | | |
| − | Fig. 2.23:
| |
| − | Strain hardening of
| |
| − | AuCu14Pt9Ag4
| |
| − | by cold working
| |
| − | | |
| − | Fig. 2.24:
| |
| − | Precipitation
| |
| − | hardening of
| |
| − | AuCu14Pt9Ag4
| |
| − | at different
| |
| − | hardening
| |
| − | temperatures
| |
| − | after 50%
| |
| − | cold working
| |
| − | | |
| − | Table 2.4: Contact and Switching Properties of Gold and Gold Alloys
| |
| − | | |
| − | Table 2.5: Application Examples and Forms of Gold and Gold Alloys
| |
| − | | |
| − | ===2.3 Platinum Metal Based Materials===
| |
| − | | |
| − | The platinum group metals include the elements Pt, Pd, Rh, Ru, Ir, and Os (Table
| |
| − | 2.6). For electrical contacts platinum and palladium have practical significance
| |
| − | as base alloy materials and ruthenium and iridium are used as alloying components.
| |
| − | Pt and Pd have similar corrosion resistance as gold but because of their
| |
| − | catalytical properties they tend to polymerize adsorbed organic vapors on contact
| |
| − | surfaces. During frictional movement between contact surfaces the polymerized
| |
| − | compounds known as “brown powder” are formed which can lead to significantly
| |
| − | increase in contact resistance. Therefore Pt and Pd are typically used as alloys and
| |
| − | not in their pure form for electrical contact applications.
| |
| − | | |
| − | Rhodium is not used as a solid contact material but is applied for example as a
| |
| − | electroplated layer in sliding contact systems. Ruthenium is mostly used as an alloying
| |
| − | component in the material PdRu15. The metals osmium and iridium have no practical
| |
| − | applications in electrical contacts.
| |
| − | | |
| − | Since Pd was for the longest time rather stable in price it was looked at as a substitute
| |
| − | for the more expensive gold. This was followed by a steep increase in the Pd price
| |
| − | which caused a significant reduction in its use in electrical contacts. Today (2011) the
| |
| − | Pd price again is lower than that of gold.
| |
| − | | |
| − | Alloys of Pt with Ru, Ir, Ni, and W were widely used in electromechanical components
| |
| − | in the telecommunication industry and in heavy duty automotive breaker points (Table
| |
| − | 2.7). Today these components have been replaced in many applications by solid
| |
| − | state technology and the usage of these materials is greatly reduced. Pd alloys
| |
| − | however have a more significant importance. PdCu15 is widely used for example in
| |
| − | automotive flasher relays. Because of their resistance to sulfide formation PdAg alloys
| |
| − | are applied in various relay designs. The ability to thermally precipitation harden some
| |
| − | multi component alloys based on PdAgAuPt they find special usage in wear resistant
| |
| − | sliding contact applications. Pd44Ag38Cu15PtAuZn is a standard alloy in this group.
| |
| − | | |
| − | Platinum and palladium alloys are mainly used similar to the gold based materials in
| |
| − | the form of welded wire and profile segments but rarely as contact rivets. Because of
| |
| − | the high precious metal prices joining technologies are used that allow the most
| |
| − | economic application of the contact alloy in the area where functionally needed.
| |
| − | Because of their resistance to material transfer they are used for DC applications and
| |
| − | due to their higher arc erosion resistance they are applied for medium electrical loads
| |
| − | up to about 30W in relays and switches (Table 2.10). Multi-component alloys based
| |
| − | on Pd with higher hardness and wear resistance are mainly used as spring arms in
| |
| − | sliding contact systems and DC miniature motors.
| |
| − | | |
| − | Table 2.6: Properties, Production Processes, and Application Forms for Platinum Metals
| |
| − | | |
| − | Table 2.7: Physical Properties of the Platinum Metals and their Alloys
| |
| − | | |
| − | Table 2.8: Mechanical Properties of the Platinum Metals and their Alloys
| |
| − | | |
| − | Fig. 2.25:
| |
| − | Influence of 1-
| |
| − | 20 atom% of
| |
| − | different additive
| |
| − | metals on the
| |
| − | electrical
| |
| − | resistivity p of
| |
| − | platinum
| |
| − | (Degussa)
| |
| − | | |
| − | Fig. 2.26:
| |
| − | Influence of 1-22 atom% of different
| |
| − | additive metals on the electrical
| |
| − | resistivity
| |
| − | p of palladium
| |
| − | | |
| − | Fig. 2.27:
| |
| − | Phase diagram of
| |
| − | platinum-iridium
| |
| − | | |
| − | Fig. 2.28:
| |
| − | Phase diagram of
| |
| − | platinum-nickel
| |
| − | | |
| − | Fig. 2.29:
| |
| − | Phase diagram
| |
| − | of platinum-tungsten
| |
| − | | |
| − | Fig. 2.30:
| |
| − | Phase diagram of
| |
| − | palladium-copper
| |
| − | | |
| − | Fig. 2.31:
| |
| − | Strain
| |
| − | hardening
| |
| − | of Pt by cold
| |
| − | working
| |
| − | | |
| − | Fig. 2.32:
| |
| − | Softening of Pt after
| |
| − | annealing for 0.5 hrs
| |
| − | after 80%
| |
| − | cold working
| |
| − | | |
| − | Fig. 2.33:
| |
| − | Strain hardening of PtIr5
| |
| − | by cold working
| |
| − | | |
| − | Fig. 2.34:
| |
| − | Softening of PtIr5 after annealing for 1 hr
| |
| − | after different degrees of cold working
| |
| − | | |
| − | Fig. 2.35:
| |
| − | Strain hardening
| |
| − | of PtNi8 by cold working
| |
| − | | |
| − | Fig. 2.36:
| |
| − | Softening of PtNi8 after
| |
| − | annealing
| |
| − | for 1 hr after
| |
| − | 80% cold working
| |
| − | | |
| − | Fig. 2.37:
| |
| − | Strain hardening
| |
| − | of PtW5 by cold working
| |
| − | | |
| − | Fig. 2.38:
| |
| − | Softening
| |
| − | of PtW5 after
| |
| − | annealing for 1hr
| |
| − | after 80% cold
| |
| − | working
| |
| − | | |
| − | Fig. 2.39:
| |
| − | Strain hardening
| |
| − | of Pd 99.99 by cold working
| |
| − | | |
| − | Fig. 2.40:
| |
| − | Strain hardening
| |
| − | of PdCu15 by cold working
| |
| − | | |
| − | Fig. 2.41:
| |
| − | Softening
| |
| − | of PdCu15 after
| |
| − | annealing
| |
| − | for 0.5 hrs
| |
| − | | |
| − | Fig. 2.42:
| |
| − | Strain hardening
| |
| − | of PdCu40 by cold working
| |
| − | | |
| − | Fig. 2.43:
| |
| − | Softening
| |
| − | of PdCu40
| |
| − | after annealing
| |
| − | for 0.5 hrs after 80%
| |
| − | cold working
| |
| − | | |
| − | Fig. 2.44:
| |
| − | Electrical resistivity p
| |
| − | of PdCu alloys with and without an
| |
| − | annealing step for forming an ordered
| |
| − | phase
| |
| − | | |
| − | Table 2.9: Contact and Switching Properties
| |
| − | of the Platinum Metals and their Alloys
| |
| − | | |
| − | Table 2.10: Application Examples and Form
| |
| − | of Supply for Platinum Metals and their Alloys
| |
| − | | |
| − | ===2.4 Silver Based Materials===
| |
| − | | |
| − | ===2.4.1 Pure Silver===
| |
| − | Pure silver (also called fine silver) exhibits the highest electrical and thermal
| |
| − | conductivity of all metals. It is also resistant against oxidation. Major disadvantages
| |
| − | are its low mechanical wear resistance, the low softening temperature,
| |
| − | and especially its strong affinity to sulfur and sulfur compounds. In the presence
| |
| − | of sulfur and sulfur containing compounds brownish to black silver sulfide layer
| |
| − | are formed on its surface. These can cause increased contact resistance or
| |
| − | even total failure of a switching device if they are not mechanically, electrically,
| |
| − | or thermally destroyed. Other weaknesses of silver contacts are the tendency to
| |
| − | weld under the influence of over-currents and the low resistance against
| |
| − | material transfer when switching DC loads. In humid environments and under
| |
| − | the influence of an electrical field silver can creep (silver migration) and cause
| |
| − | electrical shorting between adjacent current paths.
| |
| − | | |
| − | Table 2.11 shows the typically available quality grades of silver. In certain
| |
| − | economic areas, i.e. China, there are additional grades with varying amounts of
| |
| − | impurities available on the market. In powder form silver is used for a wide
| |
| − | variety of silver based composite contact materials. Different manufacturing
| |
| − | processes result in different grades of Ag powder as shown in Table 2.12.
| |
| − | additional properties of silver powders and their usage are described
| |
| − | in chapter 8.1.
| |
| − | Semi-finished silver materials can easily be warm or cold formed and can be
| |
| − | clad to the usual base materials. For attachment of silver to contact carrier
| |
| − | materials welding of wire or profile cut-offs and brazing are most widely applied.
| |
| − | Besides these mechanical processes such as wire insertion (wire staking) and
| |
| − | the riveting (staking) of solid or composite contact rivets are used in the
| |
| − | manufacture of contact components.
| |
| − | | |
| − | Contacts made from fine silver are applied in various electrical switching
| |
| − | devices such as relays, pushbuttons, appliance and control switches for
| |
| − | currents < 2 A (Table 2.16). Electroplated silver coatings are widely used to
| |
| − | reduce the contact resistance and improve the brazing behavior of other contact
| |
| − | materials and components.
| |
| − | | |
| − | Table 2.11: Overview of the Most Widely Used Silver Grades
| |
| − | | |
| − | Table 2.12: Quality Criteria of Differently Manufactured Silver Powders
| |
| − | | |
| − | Fig. 2.45:
| |
| − | Strain hardening
| |
| − | of Ag 99.95 by cold working
| |
| − | | |
| − | Fig. 2.46:
| |
| − | Softening of Ag 99.95
| |
| − | after annealing for 1 hr after different
| |
| − | degrees of strain hardening
| |
| − | | |
| − | ===2.4.2 Silver Alloys===
| |
| − | To improve the physical and contact properties of fine silver melt-metallurgical
| |
| − | produced silver alloys are used (Table 2.13). By adding metal components the
| |
| − | mechanical properties such as hardness and tensile strength as well as typical
| |
| − | contact properties such as erosion resistance, and resistance against material
| |
| − | transfer in DC circuits are increased (Table 2.14). On the other hand however,
| |
| − | other properties such as electrical conductivity and chemical corrosion
| |
| − | resistance can be negatively impacted by alloying (Figs. 2.47 and 2.48).
| |
| − | | |
| − | ===2.4.2.1 Fine-Grain Silver===
| |
| − | Fine-Grain Silver (ARGODUR-Spezial) is defined as a silver alloy with an addition
| |
| − | of 0.15 wt% of Nickel. Silver and nickel are not soluble in each other in solid
| |
| − | form. In liquid silver only a small amount of nickel is soluble as the phase diagram
| |
| − | (Fig. 2.51) illustrates. During solidification of the melt this nickel addition gets
| |
| − | finely dispersed in the silver matrix and eliminates the pronounce coarse grain
| |
| − | growth after prolonged influence of elevated temperatures (Figs. 2.49 and 2.50.
| |
| − | | |
| − | Fine-grain silver has almost the same chemical corrosion resistance as fine
| |
| − | silver. Compared to pure silver it exhibits a slightly increased hardness and
| |
| − | tensile strength (Table 2.14). The electrical conductivity is just slightly decreased
| |
| − | by this low nickel addition. Because of its significantly improved contact
| |
| − | properties fine grain silver has replaced pure silver in many applications.
| |
| − | | |
| − | ===2.4.2.2 Hard-Silver Alloys===
| |
| − | Using copper as an alloying component increases the mechanical stability of
| |
| − | silver significantly. The most important among the binary AgCu alloys is that of
| |
| − | AgCu3, known in europe also under the name of hard-silver. This material still
| |
| − | has a chemical corrosion resistance close to that of fine silver. In comparison to
| |
| − | pure silver and fine-grain silver AgCu3 exhibits increased mechanical strength
| |
| − | as well as higher arc erosion resistance and mechanical wear resistance
| |
| − | (Table 2.14).
| |
| − | | |
| − | Increasing the Cu content further also increases the mechanical strength of
| |
| − | AgCu alloys and improves arc erosion resistance and resistance against
| |
| − | material transfer while at the same time however the tendency to oxide formation
| |
| − | becomes detrimental. This causes during switching under arcing conditions an
| |
| − | increase in contact resistance with rising numbers of operation. In special
| |
| − | applications where highest mechanical strength is recommended and a reduced
| |
| − | chemical resistance can be tolerated, the eutectic AgCu alloy with 28 wt% of
| |
| − | copper (Fig. 2.52) is used. AgCu10 also known as coin silver has been
| |
| − | replaced in many applications by composite silver-based materials while sterling
| |
| − | silver (AgCu7.5) has never extended its important usage from decorative table
| |
| − | wear and jewelry to industrial applications in electrical contacts.
| |
| − | | |
| − | Besides these binary alloys, ternary AgCuNi alloys are used in electrical contact
| |
| − | applications. From this group the material ARGODUR 27, an alloy of 98 wt% Ag
| |
| − | with a 2 wt% Cu and nickel addition has found practical importance close to that
| |
| − | of AgCu3. This material is characterized by high resistance to oxidation and low
| |
| − | tendency to re-crystallization during exposure to high temperatures. Besides
| |
| − | high mechanical stability this AgCuNi alloy also exhibits a strong resistance
| |
| − | against arc erosion. Because of its high resistance against material transfer the
| |
| − | alloy AgCu24.5Ni0.5 has been used in the automotive industry for an extended
| |
| − | time in the North American market. Caused by miniaturization and the related
| |
| − | reduction in available contact forces in relays and switches this material has
| |
| − | been replaced widely because of its tendency to oxide formation.
| |
| − | | |
| − | The attachment methods used for the hard silver materials are mostly close to
| |
| − | those applied for fine silver and fine grain silver.
| |
| − | | |
| − | Hard-silver alloys are widely used for switching applications in the information
| |
| − | and energy technology for currents up to 10 A, in special cases also for higher
| |
| − | current ranges (Table 2.16).
| |
| − | | |
| − | Dispersion hardened alloys of silver with 0.5 wt% MgO and NiO (ARGODUR 32)
| |
| − | are produced by internal oxidation. While the melt-metallurgical alloy is easy to
| |
| − | cold-work and form the material becomes very hard and brittle after dispersion
| |
| − | hardening. Compared to fine silver and hard-silver this material has a greatly
| |
| − | improved temperature stability and can be exposed to brazing temperatures up
| |
| − | to 800°C without decreasing its hardness and tensile strength.
| |
| − | Because of these mechanical properties and its high electrical conductivity
| |
| − | | |
| − | Table 2.13: Physical Properties of Silver and Silver Alloys
| |
| − | | |
| − | ARGODUR 32 is mainly used in the form of contact springs that are exposed to
| |
| − | high thermal and mechanical stresses in relays, and contactors for aeronautic
| |
| − | applications.
| |
| − | | |
| − | Fig. 2.47:
| |
| − | Influence of 1-10 atom% of different
| |
| − | alloying metals on the electrical resistivity of
| |
| − | silver
| |
| − | | |
| − | Fig. 2.48:
| |
| − | Electrical resistivity p
| |
| − | of AgCu alloys with 0-20 weight% Cu
| |
| − | in the soft annealed
| |
| − | and tempered stage
| |
| − | a) Annealed and quenched
| |
| − | b) Tempered at 280°C
| |
| − | | |
| − | Fig. 2.49: Coarse grain micro structure
| |
| − | of Ag 99.97 after 80% cold working
| |
| − | and 1 hr annealing at 600°C
| |
| − | | |
| − | Fig. 2.50: Fine grain microstructure
| |
| − | of AgNi0.15 after 80% cold working
| |
| − | and 1 hr annealing at 600°C
| |
| − | | |
| − | Fig. 2.51:
| |
| − | Phase diagram
| |
| − | of silver-nickel
| |
| − | | |
| − | Fig. 2.52:
| |
| − | Phase diagram
| |
| − | of silver-copper
| |
| − | | |
| − | Fig. 2.53:
| |
| − | Phase diagram of
| |
| − | silver-cadmium
| |
| − | | |
| − | Table 2.14: Mechanical Properties of Silver and Silver Alloys
| |
| − | | |
| − | Fig. 2.54:
| |
| − | Strain hardening
| |
| − | of AgCu3
| |
| − | by cold working
| |
| − | | |
| − | Fig. 2.55:
| |
| − | Softening of AgCu3
| |
| − | after annealing for 1 hr
| |
| − | after 80% cold working
| |
| − | | |
| − | Fig. 2.56:
| |
| − | Strain hardening of AgCu5 by cold
| |
| − | working
| |
| − | | |
| − | Fig. 2.57:
| |
| − | Softening of AgCu5 after
| |
| − | annealing for 1 hr after 80% cold
| |
| − | working
| |
| − | | |
| − | Fig. 2.58:
| |
| − | Strain hardening of AgCu 10
| |
| − | by cold working
| |
| − | | |
| − | Fig. 2.59:
| |
| − | Softening of AgCu10 after
| |
| − | annealing for 1 hr after 80% cold
| |
| − | working
| |
| − | | |
| − | Fig. 2.60:
| |
| − | Strain hardening of AgCu28 by
| |
| − | cold working
| |
| − | | |
| − | Fig. 2.61:
| |
| − | Softening of AgCu28
| |
| − | after annealing for 1 hr after
| |
| − | 80% cold working
| |
| − | | |
| − | Fig. 2.62:
| |
| − | Strain hardening of AgNi0.15
| |
| − | by cold working
| |
| − | | |
| − | Fig. 2.63:
| |
| − | Softening of AgNi0.15
| |
| − | after annealing for 1 hr after 80%
| |
| − | cold working
| |
| − | | |
| − | Fig. 2.64:
| |
| − | Strain hardening of
| |
| − | ARGODUR 27
| |
| − | by cold working
| |
| − | | |
| − | Fig. 2.65:
| |
| − | Softening
| |
| − | of ARGODUR 27 after annealing
| |
| − | for 1 hr after 80% cold working
| |
| − | | |
| − | Table 2.15: Contact and Switching Properties of Silver and Silver Alloys
| |
| − | | |
| − | Table 2.16: Application Examples and Forms of Supply for Silver and Silver Alloys
| |
| − | | |
| − | ===2.4.2.3 Silver-Palladium Alloys===
| |
| − | The addition of 30 wt% Pd increases the mechanical properties as well as the
| |
| − | resistance of silver against the influence of sulfur and sulfur containing
| |
| − | compounds significantly (Tables 2.17 and 2.18).
| |
| − | Alloys with 40-60 wt% Pd have an even higher resistance against silver sulfide
| |
| − | formation. At these percentage ranges however the catalytic properties of
| |
| − | palladium can influence the contact resistance behavior negatively. The
| |
| − | formability also decreases with increasing Pd contents.
| |
| − | | |
| − | AgPd alloys are hard, arc erosion resistant, and have a lower tendency towards
| |
| − | material transfer under DC loads (Table 2.19). On the other hand the electrical
| |
| − | conductivity is decreased at higher Pd contents. The ternary alloy AgPd30Cu5
| |
| − | has an even higher hardness which makes it suitable for use in sliding contact
| |
| − | systems.
| |
| − | | |
| − | AgPd alloys are mostly used in relays for the switching of medium to higher loads
| |
| − | (>60V, >2A) as shown in Table 2.20. Because of the high palladium price these
| |
| − | formerly solid contacts have been widely replaced by multi-layer designs such
| |
| − | as AgNi0.15 or AgNi10 with a thin Au surface layer. A broader field of application
| |
| − | for AgPd alloys remains in the wear resistant sliding contact systems.
| |
| − | | |
| − | Fig. 2.66: Phase diagram of silver-palladium
| |
| − | | |
| − | Fig. 2.67:
| |
| − | Strain hardening
| |
| − | of AgPd30 by cold working
| |
| − | | |
| − | Fig. 2.68:
| |
| − | Strain hardening
| |
| − | of AgPd50 by cold working
| |
| − | | |
| − | Fig. 2.69:
| |
| − | Strain hardening
| |
| − | of AgPd30Cu5
| |
| − | by cold working
| |
| − | | |
| − | Fig. 2.70:
| |
| − | Softening of AgPd30, AgPd50,
| |
| − | and AgPd30Cu5 after annealing of 1 hr
| |
| − | after 80% cold working
| |
| − | | |
| − | Table 2.17: Physical Properties of Silver-Palladium Alloys
| |
| − | | |
| − | Table 2.18: Mechanical Properties of Silver-Palladium Alloys
| |
| − | | |
| − | Table 2.19: Contact and Switching Properties of Silver-Palladium Alloys
| |
| − | | |
| − | Table 2.20: Application Examples and Forms of Suppl for Silver-Palladium Alloys
| |
| − | | |
| − | ===2.4.3 Silver Composite Materials===
| |
| − | | |
| − | ===2.4.3.1 Silver-Nickel (SINIDUR) Materials===
| |
| − | Since silver and nickel are not soluble in each other in solid form and in the liquid
| |
| − | phase have only very limited solubility silver nickel composite materials with
| |
| − | higher Ni contents can only be produced by powder metallurgy. During extrusion
| |
| − | of sintered Ag/Ni billets into wires, strips and rods the Ni particles embedded in
| |
| − | the Ag matrix are stretched and oriented in the microstructure into a pronounced
| |
| − | fiber structure (Figs. 2.75. and 2.76)
| |
| − | | |
| − | The high density produced during hot extrusion aids the arc erosion resistance
| |
| − | of these materials (Tables 2.21 and 2.22). The typical application of Ag/Ni
| |
| − | contact materials is in devices for switching currents of up to 100A (Table 2.24).
| |
| − | In this range they are significantly more erosion resistant than silver or silver
| |
| − | alloys. In addition they exhibit with nickel contents <20 wt% a low and over their
| |
| − | operational lifetime consistent contact resistance and good arc moving
| |
| − | properties. In DC applications Ag/Ni materials exhibit a relatively low tendency
| |
| − | of material transfer distributed evenly over the contact surfaces (Table 2.23).
| |
| − | | |
| − | Typically Ag/Ni (SINIDUR) materials are usually produced with contents of 10-40
| |
| − | wt% Ni. The most widely used materials SINIDUR 10 and SINIDUR 20- and also
| |
| − | SINIDUR 15, mostly used in north america-, are easily formable and applied by
| |
| − | cladding (Figs. 2.71-2.74). They can be, without any additional welding aids,
| |
| − | economically welded and brazed to the commonly used contact carrier
| |
| − | materials.
| |
| − | The (SINIDUR) materials with nickel contents of 30 and 40 wt% are used in
| |
| − | switching devices requiring a higher arc erosion resistance and where increases
| |
| − | in contact resistance can be compensated through higher contact forces.
| |
| − | | |
| − | The most important applications for Ag/Ni contact materials are typically in
| |
| − | relays, wiring devices, appliance switches, thermostatic controls, auxiliary
| |
| − | switches, and small contactors with nominal currents >20A (Table 2.24).
| |
| − | | |
| − | Table 2.21: Physical Properties of Silver-Nickel (SINIDUR) Materials
| |
| − | | |
| − | Table 2.22: Mechanical Properties of Silver-Nickel (SINIDUR) Materials
| |
| − | | |
| − | Fig. 2.71:
| |
| − | Strain hardening
| |
| − | of Ag/Ni 90/10 by cold working
| |
| − | | |
| − | Fig. 2.72:
| |
| − | Softening of Ag/Ni 90/10
| |
| − | after annealing
| |
| − | for 1 hr after 80% cold working
| |
| − | | |
| − | Fig. 2.73:
| |
| − | Strain hardening
| |
| − | of Ag/Ni 80/20 by cold working
| |
| − | | |
| − | Fig. 2.74:
| |
| − | Softening of Ag/Ni 80/20
| |
| − | after annealing
| |
| − | for 1 hr after 80% cold working
| |
| − | | |
| − | Fig. 2.75: Micro structure of Ag/Ni 90/10 a) perpendicular to the extrusion direction
| |
| − | b) parallel to the extrusion direction
| |
| − | | |
| − | Fig. 2.76: Micro structure of Ag/Ni 80/20 a) perpendicular to the extrusion direction
| |
| − | b) parallel t o the extrusion direction
| |
| − | | |
| − | Table 2.23: Contact and Switching Properties of Silver-Nickel (SINIDUR) Materials
| |
| − | | |
| − | Table 2.24: Application Examples and Forms of Supply
| |
| − | for Silver-Nickel (SINIDUR) Materials
| |
| − | | |
| − | ===2.4.3.2: Silver-Metal Oxide Materials Ag/CdO, Ag/SnO , Ag/ZnO===
| |
| − | The family of silver-metal oxide contact materials includes the material groups:
| |
| − | silver-cadmium oxide (DODURIT CdO), silver-tin oxide (SISTADOX), and silverzinc
| |
| − | oxide (DODURIT ZnO). Because of their very good contact and switching
| |
| − | properties like high resistance against welding, low contact resistance, and high
| |
| − | arc erosion resistance, silver-metal oxides have gained an outstanding position
| |
| − | in a broad field of applications. They mainly are used in low voltage electrical
| |
| − | switching devices like relays, installation and distribution switches, appliances,
| |
| − | industrial controls, motor controls, and protective devices (Table 2.13).
| |
| − | | |
| − | *Silver-cadmium oxide (DODURIT CdO) materials
| |
| − | | |
| − | Silver-cadmium oxide (DODURIT CdO) materials with 10-15 wt% are produced
| |
| − | by both, internal oxidation and powder metallurgical methods (Table 2.25).
| |
| − | | |
| − | The manufacturing of strips and wires by internal oxidation starts with a molten
| |
| − | alloy of silver and cadmium. During a heat treatment below it's melting point in a
| |
| − | oxygen rich atmosphere in such a homogeneous alloy the oxygen diffuses from
| |
| − | the surface into the bulk of the material and oxidizes the Cd to CdO in a more or
| |
| − | less fine particle precipitation inside the Ag matrix. The CdO particles are rather
| |
| − | fine in the surface area and are becoming larger further away towards the center
| |
| − | of the material (Fig. 2.83).
| |
| − | | |
| − | During the manufacturing of Ag/CdO contact material by internal oxidation the
| |
| − | processes vary depending on the type of semi-finished material.
| |
| − | For Ag/CdO wires a complete oxidation of the AgCd wire is performed, followed
| |
| − | by wire-drawing to the required diameter (Figs. 2.77 and 2.78). The resulting
| |
| − | material is used for example in the production of contact rivets. For Ag/CdO strip
| |
| − | materials two processes are commonly used: Cladding of an AgCd alloy strip
| |
| − | with fine silver followed by complete oxidation results in a strip material with a
| |
| − | small depletion area in the center of it's thickness and a Ag backing suitable for
| |
| − | easy attachment by brazing (sometimes called “Conventional Ag/CdO”). Using
| |
| − | a technology that allows the partial oxidation of a dual-strip AgCd alloy material
| |
| − | in a higher pressure pure oxygen atmosphere yields a composite Ag/CdO strip
| |
| − | material that has besides a relatively fine CdO precipitation also a easily brazable
| |
| − | AgCd alloy backing (Fig. 2.85). These materials (DODURIT CdO ZH) are mainly
| |
| − | used as the basis for contact profiles and contact tips.
| |
| − | | |
| − | During powder metallurgical production the powder mixed made by different
| |
| − | processes are typically converted by pressing, sintering and extrusion to wires
| |
| − | and strips. The high degree of deformation during hot extrusion produces a
| |
| − | uniform and fine dispersion of CdO particles in the Ag matrix while at the same
| |
| − | time achieving a high density which is advantageous for good contact properties
| |
| − | (Fig. 2.84). To obtain a backing suitable for brazing, a fine silver layer is applied
| |
| − | by either com-pound extrusion or hot cladding prior to or right after the extrusion
| |
| − | (Fig. 2.86).
| |
| − | | |
| − | For larger contact tips, and especially those with a rounded shape, the single tip
| |
| − | Press-Sinter-Repress process (PSR) offers economical advantages. The
| |
| − | powder mix is pressed in a die close to the final desired shape, the “green” tips
| |
| − | are sintered, and in most cases the repress process forms the final exact shape
| |
| − | while at the same time increasing the contact density and hardness.
| |
| | | | |
| − | Using different silver powders and minor additives for the basic Ag and CdO
| + | ==Gold Based Materials== |
| − | starting materials can help influence certain contact properties for specialized
| |
| − | applications.
| |
| | | | |
| − | Fig. 2.77:
| + | Pure Gold is besides Platinum the chemically most stable of all precious metals. In its pure form, it is not very suitable for use as a contact material in electromechanical devices because of its tendency to stick and cold-weld at even low contact forces. In addition, it is not hard or strong enough to resist mechanical wear and exhibits high material losses under electrical arcing loads. This limits its use in form of thin electroplated or vacuum deposited layers. |
| − | Strain hardening of internally oxidized
| |
| − | Ag/CdO 90/10 by cold working
| |
| | | | |
| − | Fig. 2.78:
| + | Main Article: [[Gold Based Materials| Gold Based Materials]] |
| − | Softening of internally oxidized
| |
| − | Ag/CdO 90/10 after annealing
| |
| − | for 1 hr after 40% cold working
| |
| | | | |
| − | Table 2.25: Physical and Mechanical Properties as well as Manufacturing Processes and
| + | ==Platinum Metal Based Materials== |
| − | Forms of Supply of Extruded Silver Cadmium Oxide
| |
| − | (DODURIT CdO) Contact Materials
| |
| | | | |
| − | Fig. 2.79:
| + | The platinum group metals include the elements Pt, Pd, Rh, Ru, Ir and Os ([[Platinum_Metal_Based_Materials|Table 1]]<!--(Table 2.6)-->). For electrical contacts, platinum and palladium have practical significance as base alloy materials and ruthenium and iridium are used as alloying components. Pt and Pd have similar corrosion resistance as gold but due to their catalytical properties, they tend to polymerize adsorbed organic vapors on contact surfaces. During frictional movement between contact surfaces, the polymerized compounds known as “brown powder” are formed, which can lead to a significant increase in contact resistance. Therefore Pt and Pd are typically used as alloys and are rather not used in their pure form for electrical contact applications. |
| − | Strain hardening of
| |
| − | Ag/CdO 90/10 P by cold working
| |
| | | | |
| − | Fig. 2.80: Softening
| + | Main Article: [[Platinum Metal Based Materials| Platinum Metal Based Materials]] |
| − | of Ag/CdO 90/10 P after annealing
| |
| − | for 1 hr after 40% cold working
| |
| | | | |
| − | Fig. 2.81:
| + | ==Silver Based Materials== |
| − | Strain hardening
| |
| − | of Ag/CdO 88/12 WP
| |
| | | | |
| − | Fig. 2.82:
| + | Main Article: [[Silver Based Materials| Silver Based Materials]] |
| − | Softening of Ag/CdO 88/12WP after annealing
| |
| − | for 1 hr after different degrees of
| |
| − | cold working
| |
| | | | |
| − | Fig. 2.83: Micro structure of Ag/CdO 90/10 i.o. a) close to surface
| + | ==Tungsten and Molybdenum Based Materials== |
| − | b) in center area
| |
| | | | |
| − | Fig. 2.84: Micro structure of Ag/CdO 90/10 P:
| + | Main Article: [[Tungsten and Molybdenum Based Materials| Tungsten and Molybdenum Based Materials]] |
| − | a) perpendicular to extrusion direction
| |
| − | b) parallel to extrusion direction
| |
| | | | |
| − | Fig. 2.85:
| + | ==Contact Materials for Vacuum Switches== |
| − | Micro structure of Ag/CdO 90/10 ZH:
| |
| − | 1) Ag/CdO layer
| |
| − | 2) AgCd backing layer
| |
| | | | |
| − | Fig. 2.86: Micro structure of AgCdO 88/12 WP: a) perpendicular to extrusion direction
| + | The low gas content contact materials are developed for the use in vacuum switching devices. |
| − | b) parallel to extrusion direction
| |
| | | | |
| − | *Silver–tin oxide(SISTADOX)materials
| + | Main Article: [[Contact Materials for Vacuum Switches| Contact Materials for Vacuum Switches]] |
| − | Over the past years, many Ag/CdO contact materials have been replaced by
| |
| − | Ag/SnO<sub>2</sub> based materials with 2-14 wt% SnO<sub>2</sub> because of the toxicity of
| |
| − | Cadmium. This changeover was further favored by the fact that Ag/SnO<sub>2</sub>
| |
| − | contacts quite often show improved contact and switching properties such as
| |
| − | lower arc erosion, higher weld resistance, and a significant lower tendency
| |
| − | towards material transfer in DC switching circuits ''(Table 2.30)''. Ag/SnO<sub>2</sub>
| |
| − | materials have been optimized for a broad range of applications by other metal
| |
| − | oxide additives and modification in the manufacturing processes that result in
| |
| − | different metallurgical, physical and electrical properties ''(Table 2.29)''.
| |
| | | | |
| − | Manufacturing of Ag/SnO<sub>2</sub> by ''internal oxidation'' is possible in principle, but
| + | ==References== |
| − | during heat treatment of alloys containing > 5 wt% of tin in oxygen, dense oxide
| |
| − | layers formed on the surface of the material prohibit the further diffusion of
| |
| − | oxygen into the bulk of the material. By adding Indium or Bismuth to the alloy the
| |
| − | internal oxidation is possible and results in materials that typically are rather hard
| |
| − | and brittle and may show somewhat elevated contact resistance and is limited
| |
| − | to applications in relays. To make a ductile material with fine oxide dispersion
| |
| − | (SISTADOX TOS F) ''(Fig. 2.114)'' it is necessary to use special process variations
| |
| − | in oxidation and extrusion which lead to materials with improved properties in
| |
| − | relays. Adding a brazable fine silver layer to such materials results in a semifinished
| |
| − | material suitable for the manufacture as smaller weld profiles
| |
| − | (SISTADOX WTOS F) ''(Fig. 2.116)''. Because of their resistance to material
| |
| − | transfer and low arc erosion these materials find for example a broader
| |
| − | application in automotive relays ''(Table 2.31)''.
| |
| | | | |
| − | ''Powder metallurgy'' plays a significant role in the manufacturing of Ag/SnO<sub>2</sub>
| + | Vinaricky, E.(Hrsg.): Elektrische Kontakte, Werkstoffe und Anwendungen. |
| − | contact materials. Besides SnO<sub>2</sub> a smaller amount (<1 wt%) of one or more
| + | Springer-Verlag, Berlin, Heidelberg etc. 2002 |
| − | other metal oxides such as WO<sub>3</sub>, MoO<sub>3</sub>, CuO and/or Bi<sub>2</sub>O<sub>3</sub> are added. These
| |
| − | additives improve the wettability of the oxide particles and increase the viscosity
| |
| − | of the Ag melt. They also provide additional benefits to the mechanical and
| |
| − | arcing contact properties of materials in this group ''(Table 2.26)''.
| |
| | | | |
| − | In the manufacture the initial powder mixes different processes are applied
| + | Lindmayer, M.: Schaltgeräte-Grundlagen, Aufbau, Wirkungsweise. |
| − | which provide specific advantages of the resulting materials in respect to their
| + | Springer-Verlag, Berlin, Heidelberg, New York, Tokio, 1987 |
| − | contact properties ''(Figs. 2.87 – 2.119)''. Some of them are described here as
| |
| − | follows:
| |
| − | :'''a) Powder blending from single component powders''' <br> In this common process all components including additives that are part of the powder mix are blended as single powders. The blending is usually performed in the dry stage in blenders of different design.
| |
| | | | |
| − | :'''b) Powder blending on the basis of doped powders''' <br> For incorporation of additive oxides in the SnO<sub>2</sub> powder the reactive spray process (RSV) has shown advantages. This process starts with a waterbased solution of the tin and other metal compounds. This solution is nebulized under high pressure and temperature in a reactor chamber. Through the rapid evaporation of the water each small droplet is converted into a salt crystal and from there by oxidation into a tin oxide particle in which the additive metals are distributed evenly as oxides. The so created doped AgSnO2 powder is then mechanically mixed with silver powder. | + | Rau, G.: Metallische Verbundwerkstoffe. Werkstofftechnische |
| | + | Verlagsgesellschaft, Karlsruhe 1977 |
| | | | |
| − | :'''c) Powder blending based on coated oxide powders''' <br> In this process tin oxide powder is blended with lower meting additive oxides such as for example Ag<sub>2</sub> MoO<sub>4</sub> and then heat treated. The SnO<sub>2</sub> particles are coated in this step with a thin layer of the additive oxide. | + | Schreiner, H.: Pulvermetallurgie elektrischer Kontakte. Springer-Verlag |
| | + | Berlin, Göttingen, Heidelberg, 1964 |
| | | | |
| − | :'''d) Powder blending based on internally oxidized alloy powders''' <br> A combination of powder metallurgy and internal oxidation this process starts with atomized Ag alloy powder which is subsequently oxidized in pure oxygen. During this process the Sn and other metal components are transformed to metal oxide and precipitated inside the silver matrix of each powder particle. | + | Hansen. M.; Anderko, K.: Constitution of Binary Alloys. New York: |
| | + | Mc Graw-Hill, 1958 |
| | | | |
| − | :'''e) Powder blending based on chemically precipitated compound powders''' <br> A silver salt solution is added to a suspension of for example SnO<sub>2</sub> together with a precipitation agent. In a chemical reaction silver and silver oxide respectively are precipitated around the additive metal oxide particles who act as crystallization sites. Further chemical treatment then reduces the silver oxide with the resulting precipitated powder being a mix of Ag and SnO<sub>2</sub>. | + | Shunk, F.A.: Constitution of Binary Alloy. 2 Suppl. New York; Mc Graw-Hill, 1969 |
| | | | |
| − | Further processing of these differently produced powders follows the
| + | Edelmetall-Taschenbuch. ( Herausgeber Degussa AG, Frankfurt a. M.), |
| − | conventional processes of pressing, sintering and hot extrusion to wires and
| + | Heidelberg, Hüthig-Verlag, 1995 |
| − | strips. From these contact parts such as contact rivets and tips are
| |
| − | manufactured. To obtain a brazable backing the same processes as used for
| |
| − | Ag/CdO are applied. As for Ag/CdO, larger contact tips can also be
| |
| − | manufactured more economically using the press-sinter-repress (PSR) process
| |
| − | ''(Table 2.27).''
| |
| | | | |
| − | Fig. 2.87:
| + | Rau, G.: Elektrische Kontakte-Werkstoffe und Technologie. Eigenverlag G. Rau |
| − | Strain hardening of
| + | GmbH & Co., Pforzheim, 1984 |
| − | Ag/SnO<sub>2</sub> 92/8 PE by cold working
| |
| | | | |
| − | Fig. 2.88:
| + | Heraeus, W. C.: Werkstoffdaten. Eigenverlag W.C. Heraeus, Hanau, 1978 |
| − | Softening of
| |
| − | Ag/SnO<sub>2</sub> 92/8 PE after annealing
| |
| − | for 1 hr after 40% cold working
| |
| | | | |
| − | Table 2.26: Physical and Mechanical Properties as well as Manufacturing Processes and
| + | Linde, J.O.: Elektrische Widerstandseigenschaften der verdünnten Legierungen |
| − | Forms of Supply of Extruded Silver-Tin Oxide (SISTADOX) Contact Materials
| + | des Kupfers, Silbers und Goldes. Lund: Hakan Ohlsson, 1938 |
| | | | |
| − | Fig. 2.89:
| + | Engineers Relay Handbook, RSIA, 2006 |
| − | Strain hardening of
| |
| − | Ag/SnO<sub>2</sub> 88/12 PE by cold working
| |
| | | | |
| − | Fig. 2.90:
| + | Großmann, H. Saeger, K. E.; Vinaricky, E.: Gold and Gold Alloys in Electrical |
| − | Softening of Ag/SnO<sub>2</sub> 88/12 PE
| + | Engineering. in: Gold, Progress in Chemistry, Biochemistry and Technology. John |
| − | after annealing for
| + | Wiley & Sons, Chichester etc, (1999) 199-236 |
| − | 1 hr after 40% cold working
| |
| | | | |
| − | Fig. 2.91:
| + | Gehlert, B.: Edelmetall-Legierungen für elektrische Kontakte. |
| − | Strain hardening of oxidized
| + | Metall 61 (2007) H. 6, 374-379 |
| − | Ag/SnO<sub>2</sub> 88/12 PW4 by cold working
| |
| | | | |
| − | Fig. 2.92:
| + | Aldinger, F.; Schnabl, R.: Edelmetallarme Kontakte für kleine Ströme. |
| − | Softening of Ag/SnO<sub>2</sub> 88/12 PW4 after
| + | Metall 37 (1983) 23-29 |
| − | annealing for 1 hr
| |
| − | after 30% cold working
| |
| | | | |
| − | Fig. 2.93:
| + | Bischoff, A.; Aldinger, F.: Einfluss geringer Zusätze auf die mechanischen |
| − | Strain hardening of
| + | Eigenschaften von Au-Ag-Pd-Legierungen. Metall 36 (1982) 752-765 |
| − | Ag/SnO<sub>2</sub> 98/2 PX | |
| − | by cold working
| |
| | | | |
| − | Fig. 2.94:
| + | Wise, E.M.: Palladium, Recovery, Properties and Uses. New York, London: |
| − | Softening of
| + | Academic Press 1968 |
| − | Ag/SnO<sub>2</sub> 98/2 PX
| |
| − | after annealing
| |
| − | for 1 hr after 80%
| |
| − | cold working
| |
| | | | |
| − | Fig 2.95:
| + | Savitskii, E.M.; Polyakova, V.P.; Tylina, M.A.: Palladium Alloys, Primary Sources. |
| − | Strain hardening
| + | New York: Publishers 1969 |
| − | of Ag/SnO<sub>2</sub> 92/8 PX
| |
| − | by cold working
| |
| | | | |
| − | Fig. 2.96:
| + | Gehlert, B.: Lebensdaueruntersuchungen von Edelmetall Kontaktwerkstoff- |
| − | Softening of
| + | Kombinationen für Schleifringübertrager. VDE-Fachbericht 61, (2005) 95-100 |
| − | Ag/SnO<sub>2</sub> 92/8 PX
| |
| − | after annealing for 1 hr
| |
| − | after 40% cold working
| |
| | | | |
| − | Fig. 2.97:
| + | Holzapfel,C.: Verschweiß und elektrische Eigenschaften von |
| − | Strain hardening of internally
| + | Schleifringübertragern. VDE-Fachbericht 67 (2011) 111-120 |
| − | oxidized
| |
| − | Ag/SnO<sub>2</sub> 88/12 TOS F
| |
| − | by cold working
| |
| | | | |
| − | Fig. 2.98:
| + | Schnabl, R.; Gehlert, B.: Lebensdauerprüfungen von Edelmetall- |
| − | Softening of
| + | Schleifkontaktwerkstoffen für Gleichstrom Kleinmotoren. |
| − | Ag/SnO<sub>2</sub> 88/12 TOS F after
| + | Feinwerktechnik & Messtechnik (1984) 8, 389-393 |
| − | annealing for 1 hr after 30%
| |
| − | cold working
| |
| | | | |
| − | Fig. 2.99:
| + | Kobayashi, T.; Koibuchi, K.; Sawa, K.; Endo, K.; Hagino, H.: A Study of Lifetime |
| − | Strain hardening of
| + | of Au-plated Slip-Ring and AgPd Brush System for Power Supply. |
| − | internally oxidized
| + | th Proc. 24 Int. Conf. on Electr. Contacts, Saint Malo, France 2008, 537-542 |
| − | Ag/SnO<sub>2</sub> 88/12P
| |
| − | by cold working
| |
| | | | |
| − | Fig. 2.100:
| + | Harmsen, U.; Saeger K.E.: Über das Entfestigungsverhalten von Silber |
| − | Softening of
| + | verschiedener Reinheiten. Metall 28 (1974) 683-686 |
| − | Ag/SnO<sub>2</sub> 88/12P
| |
| − | after annealing for 1 hr after
| |
| − | 40% cold working
| |
| | | | |
| − | Fig. 2.101:
| + | Behrens, V.; Michal, R.; Minkenberg, J.N.; Saeger, K.E.: Abbrand und |
| − | Strain hardening of
| + | Kontaktwiderstandsverhalten von Kontaktwerkstoffen auf Basis von Silber- |
| − | Ag/SnO<sub>2</sub> 88/12 WPC
| + | Nickel. e.& i. 107. Jg. (1990), 2, 72-77 |
| − | by cold working
| |
| | | | |
| − | Fig. 2.102:
| + | Behrens, V.: Silber/Nickel und Silber/Grafit- zwei Spezialisten auf dem Gebiet |
| − | Softening of Ag/SnO<sub>2</sub> 88/12 WPC after annealing
| + | der Kontaktwerkstoffe. Metall 61 (2007) H.6, 380-384 |
| − | for 1 hr after different degrees of cold working
| |
| | | | |
| − | Fig. 2.103:
| + | Rieder, W.: Silber / Metalloxyd-Werkstoffe für elektrische Kontakte, |
| − | Strain hardening of
| + | VDE - Fachbericht 42 (1991) 65-81 |
| − | Ag/SnO<sub>2</sub> 86/14 WPC
| |
| − | by cold working
| |
| | | | |
| − | Fig. 2.104:
| + | Harmsen,U.: Die innere Oxidation von AgCd-Legierungen unter |
| − | Softening of Ag/SnO<sub>2</sub> 86/14 WPC after annealing
| + | Sauerstoffdruck. |
| − | for 1 hr after different degrees of cold working
| + | Metall 25 (1991), H.2, 133-137 |
| | | | |
| − | Fig. 2.105:
| + | Muravjeva, E.M.; Povoloskaja, M.D.: Verbundwerkstoffe Silber-Zinkoxid und |
| − | Strain hardening of
| + | Silber-Zinnoxid, hergestellt durch Oxidationsglühen. |
| − | Ag/SnO<sub>2</sub> 88/12 WPD
| + | Elektrotechnika 3 (1965) 37-39 |
| − | by cold working
| |
| | | | |
| − | Fig. 2.106:
| + | Behrens, V.; Honig Th.; Kraus, A.; Michal, R.; Saeger, K.-E.; Schmidberger, R.; |
| − | Softening of Ag/SnO<sub>2</sub> 88/12 WPD after
| + | Staneff, Th.: Eine neue Generation von AgSnO<sub>2</sub> -Kontaktwerkstoffen. |
| − | annealing for 1 hr after different degrees
| + | VDE-Fachbericht 44, (1993) 99-114 |
| − | of cold working
| |
| | | | |
| − | Fig. 2.108:
| + | Braumann, P.; Lang, J.: Kontaktverhalten von Ag-Metalloxiden für den Bereich |
| − | Softening of Ag/SnO<sub>2</sub> 88/12 WPX after
| + | hoher Ströme. VDE-Fachbericht 42, (1991) 89-94 |
| − | annealing for 1 hr after different degrees
| |
| − | of cold working
| |
| | | | |
| − | Fig. 2.107:
| + | Hauner, F.; Jeannot, D.; Mc Neilly, U.; Pinard, J.: Advanced AgSnO Contact 2 |
| − | Strain hardening of
| + | th Materials for High Current Contactors. Proc. 20 Int. Conf. on Electr. Contact |
| − | Ag/SnO<sub>2</sub> 88/12 WPX
| + | Phenom., Stockholm 2000, 193-198 |
| − | by cold working
| |
| | | | |
| − | Fig. 2.109: Micro structure of Ag/SnO<sub>2</sub> 92/8 PE: a) perpendicular to extrusion direction
| + | Wintz, J.-L.; Hardy, S.; Bourda, C.: Influence on the Electrical Performances of |
| − | b) parallel to extrusion direction
| + | Assembly Process, Supports Materials and Production Means for AgSnO<sub>2</sub> . |
| | + | Proc.24<sub>th</sub> Int. Conf. on Electr. Contacts, Saint Malo, France 2008, 75-81 |
| | | | |
| − | Fig. 2.110: Micro structure of Ag/SnO<sub>2</sub> 88/12 PE: a) perpendicular to extrusion direction
| + | Behrens, V.; Honig, Th.; Kraus, A.; Michal, R.: Schalteigenschaften von |
| − | b) parallel to extrusion direction
| + | verschiedenen Silber-Zinnoxidwerkstoffen in Kfz-Relais. VDE-Fachbericht 51 |
| | + | (1997) 51-57 |
| | | | |
| − | Fig. 2.111: Micro structure of Ag/SnO<sub>2</sub> 88/12 PW: a) perpendicular to extrusion direction
| + | Schöpf, Th.: Silber/Zinnoxid und andere Silber-Metalloxidwerkstoffe in |
| − | b) parallel to extrusion direction
| + | Netzrelais. VDE-Fachbericht 51 (1997) 41-50 |
| | | | |
| − | Fig. 2.112: Micro structure of Ag/SnO<sub>2</sub> 98/2 PX: a) perpendicular to extrusion direction
| + | Schöpf, Th.; Behrens, V.; Honig, Th.; Kraus, A.: Development of Silver Zinc |
| − | b) parallel to extrusion direction
| + | th Oxide for General-Purpose Relays. Proc. 20 Int. Conf. on Electr. Contacts, |
| | + | Stockholm 2000, 187-192 |
| | | | |
| − | Fig. 2.113: Micro structure of Ag/SnO<sub>2</sub> 92/8 PX: a) perpendicular to extrusion direction
| + | Braumann, P.; Koffler, A.: Einfluss von Herstellverfahren, Metalloxidgehalt und |
| − | b) parallel to extrusion direction
| + | Wirkzusätzen auf das Schaltverhalten von Ag/SnO in Relais. 2 |
| | + | VDE-Fachbericht 59, (2003) 133-142 |
| | | | |
| − | Fig. 2.114: Micro structure of Ag/SnO<sub>2</sub> 88/12 TOS F: a) perpendicular to extrusion direction
| + | Kempf, B.; Braumann, P.; Böhm, C.; Fischer-Bühner, J.: Silber-Zinnoxid- |
| − | b) parallel to extrusion direction
| + | Werkstoffe: Herstellverfahren und Eigenschaften. Metall 61(2007) H. 6, 404-408 |
| | | | |
| − | Fig. 2.115: Micro structure of Ag/SnO<sub>2</sub> 86/14 WPC: a) perpendicular to extrusion direction
| + | Lutz, O.; Behrens, V.; Finkbeiner, M.; Honig, T.; Späth, D.: Ag/CdO-Ersatz in |
| − | b) parallel to extrusion direction, 1) AgSnO<sub>2</sub> contact layer, 2) Ag backing layer
| + | Lichtschaltern. VDE-Fachbericht 61, (2005) 165-173 |
| | | | |
| − | Fig. 2.116: Micro structure of Ag/SnO<sub>2</sub> 92/8 WTOS F: a) perpendicular to extrusion direction
| + | Lutz, O.; Behrens, V.; Wasserbäch, W.; Franz, S.; Honig, Th.; Späth, |
| − | b) parallel to extrusion direction,1) AgSnO<sub>2</sub> contact layer, 2) Ag backing layer
| + | D.; Heinrich, J.: Improved Silver/Tin Oxide Contact Materials for Automotive |
| | + | th Applications. Proc.24 Int. Conf. on Electr. Contacts, Saint Malo, France 2008, |
| | + | 88-93 |
| | | | |
| − | Fig. 2.117: Micro structure of
| + | Leung, C.; Behrens, V.: A Review of Ag/SnO Contact Materials and Arc Erosion. 2 |
| − | Ag/SnO<sub>2</sub> 88/12 WPD: parallel to extrusion direction | + | th Proc.24 Int. Conf. on Electr. Contacts, Saint Malo, France 2008, 82-87 |
| − | 1) AgSnO<sub>2</sub> contact layer, 2) Ag backing layer
| |
| | | | |
| − | Fig. 2.118: Micro structure of
| + | Chen, Z.K.; Witter, G.J.: Comparison in Performance for Silver–Tin–Indium |
| − | Ag/SnO<sub>2</sub> 88/12 WPX:parallel to extrusion direction
| + | Oxide Materials Made by Internal Oxidation and Powder Metallurgy. |
| − | 1) AgSnO<sub>2</sub> contact layer, 2) Ag backing layer
| + | th Proc. 55 IEEE Holm Conf. on Electrical Contacts, Vancouver, BC, Canada, |
| | + | (2009) 167 – 176 |
| | | | |
| − | Fig. 2.119: Micro structure of Ag/SnO<sub>2</sub> 86/14 WPX: a) perpendicular to extrusion direction
| + | Roehberg, J.; Honig, Th.; Witulski, N.; Finkbeiner, M.; Behrens, V.: Performance |
| − | b) parallel to extrusion direction, 1) AgSnO<sub>2</sub> contact layer, 2) Ag backing layer
| + | of Different Silver/Tin Oxide Contact Materials for Applications in Low Voltage |
| | + | th Circuit Breakers. Proc. 55 IEEE Holm Conf. on Electrical Contacts, Vancouver, |
| | + | BC, Canada, (2009) 187 – 194 |
| | | | |
| − | Table 2.27: Physical Properties of Powder Metallurgical Silver-Metal Oxide Materials
| + | Muetzel, T.; Braumann, P.; Niederreuther, R.: Temperature Rise Behavior of |
| − | with Fine Silver Backing Produced by the Press-Sinter-Repress Process
| + | th Ag/SnO Contact Materials for Contactor Applications. Proc. 55 IEEE Holm 2 |
| | + | Conf. on Electrical Contacts, Vancouver, BC, Canada, (2009) 200 – 205 |
| | | | |
| − | *'''Silver–zinc oxide (DODURIT ZnO) materials'''
| + | Lutz, O. et al.: Silber/Zinnoxid – Kontaktwerkstoffe auf Basis der Inneren |
| − | Silver zinc oxide (DODURIT ZnO) contact materials with mostly 6 - 10 wt% oxide
| + | Oxidation fuer AC – und DC – Anwendungen. |
| − | content including other small metal oxides are produced exclusively by powder
| + | VDE Fachbericht 65 (2009) 167 – 176 |
| − | metallurgy ''(Figs. 2.120 – 2.125)'' ''(Table 2.28)''. Adding Ag<sub>2</sub>WO<sub>4</sub> in the process b)
| |
| − | as described in the preceding chapter on Ag/SnO<sub>2</sub> has proven most effective
| |
| − | for applications in AC relays, wiring devices, and appliance controls. Just like
| |
| − | with the other Ag metal oxide materials, semi-finished materials in strip and wire
| |
| − | form are used to manufacture contact tips and rivets.
| |
| − | Because of their high resistance against welding and arc erosion Ag/ZnO
| |
| − | materials present an economic alternative to Cd free Ag-tin oxide contact
| |
| − | materials ''(Tables 2.30 and 2.31)''.
| |
| | | | |
| − | Table 2.28: Physical and Mechanical Properties as well as Manufacturing Processes and
| + | Harmsen, U.; Meyer, C.L.: Mechanische Eigenschaften stranggepresster Silber- |
| − | Forms of Supply of Extruded Silver-Zinc Oxide (DODURIT ZnO) Contact
| + | Graphit-Verbundwerkstoffe. Metall 21 (1967), 731-733 |
| | | | |
| − | Fig. 2.120: Strain hardening of
| + | Behrens, V.: Mahle, E.; Michal, R.; Saeger, K.E.: An Advanced Silver/Graphite |
| − | Ag/ZnO 92/8 PW25 by cold working
| + | th Contact Material Based on Graphite Fibre. Proc. 16 Int. Conf. on Electr. |
| | + | Contacts, Loghborough 1992, 185-189 |
| | | | |
| − | Fig. 2.121: Softening of Ag/ZnO 92/8 PW25
| + | Schröder, K.-H.; Schulz, E.-D.: Über den Einfluss des Herstellungsverfahrens |
| − | after annealing for 1 hr after 30% cold working
| + | th auf das Schaltverhalten von Kontaktwerkstoffen der Energietechnik. Proc. 7 Int. |
| | + | Conf. on Electr. Contacts, Paris 1974, 38-45 |
| | | | |
| − | Fig. 2.122: Strain hardening of
| + | Mützel, T.: Niederreuther, R.: Kontaktwerkstoffe für Hochleistungsanwendungen. |
| − | Ag/ZnO 92/8 WPW25
| + | VDE-Bericht 67 (2011) 103-110 |
| − | by cold working
| |
| | | | |
| − | Fig. 2.123: Softening of
| + | Lambert, C.; Cambon, G.: The Influence of Manufacturing Conditions and |
| − | Ag/ZnO 92/8 WPW25 after annealing for
| + | Metalurgical Characteristics on the Electrical Behaviour of Silver-Graphite |
| − | 1hr after different degrees of cold working
| + | th Contact Materials. Proc. 9 Int. Conf.on Electr. Contacts, |
| | + | Chicago 1978, 401-406 |
| | | | |
| − | Fig. 2.115: Micro structure of Ag/ZnO 92/8 Pw25: a) perpendicular to extrusion direction
| + | Vinaricky, E.: Grundsätzliche Untersuchungen zum Abbrand- und |
| − | b) parallel to extrusion direction
| + | Schweißverhalten von Ag/C-Kontaktwerkstoffen. VDE-Fachbericht 47 (1995) |
| | + | 159-169 |
| | | | |
| − | Fig. 2.116: Micro structure of Ag/ZnO 92/8 WPW25:a) perpendicular to extrusion direction
| + | Agte, C.; Vacek, J.: Wolfram und Molybdän. Berlin: Akademie-Verlag 1959 |
| − | b) parallel to extrusion direction, 1) Ag/ZnO contact layer, 2) Ag backing layer
| |
| | | | |
| − | Table 2.29: Optimizing of Silver–Tin Oxide Materials Regarding their Switching
| + | Keil, A.; Meyer, C.-L.: Der Einfluß des Faserverlaufes auf die elektrische |
| − | Properties and Forming Behavior
| + | Verschleißfestigkeit von Wolfram-Kontakten. ETZ 72, (1951) 343-346 |
| | | | |
| − | Table 2.30: Contact and Switching Properties of Silver–Metal Oxide Materials
| + | Slade, P. G.: Electric Contacts for Power Interruption. A Review. Proc. 19 Int. |
| | + | Conf. on Electric Contact Phenom. Nuremberg (Germany) 1998, 239-245 |
| | | | |
| − | Table 2.31: Application Examples of Silver–Metal Oxide Materials
| + | Slade, P. G.: Variations in Contact Resistance Resulting from Oxide Formation |
| | + | and Decomposition in AgW and Ag-WC-C Contacts Passing Steady Currents |
| | + | for Long Time Periods. IEEE Trans. Components, Hybrids and Manuf. Technol. |
| | + | CHMT-9,1 (1986) 3-16 |
| | | | |
| − | ===2.4.3.3 Silver–Graphite (GRAPHOR)-Materials===
| + | Slade, P. G.: Effect of the Electric Arc and the Ambient Air on the Contact |
| − | Ag/C (GRAPHOR) contact materials are usually produced by powder metallurgy
| + | Resistance of Silver, Tungsten and Silver-Tungsten Contacts. |
| − | with graphite contents of 2 – 5 wt% ''(Table 2.32)''. The earlier typical
| + | J.Appl.Phys. 47, 8 (1976) 3438-3443 |
| − | manufacturing process of single pressed tips by pressing - sintering - repressing
| |
| − | (PSR) has been replaced in Europe for quite some time by extrusion. In North | |
| − | America and some other regions however the PSR process is still used to some
| |
| − | extend mainly for cost reasons.
| |
| | | | |
| − | The extrusion of sintered billets is now the dominant manufacturing method for
| + | Lindmayer, M.; Roth, M.: Contact Resistance and Arc-Erosion of W-Ag and |
| − | semi-finished AgC materials ''(Figs. 2.126 – 2.129)''. The hot extrusion process
| + | WC-Ag. IEEE Trans components, Hybrids and Manuf. Technol. |
| − | results in a high density material with graphite particles stretched and oriented in
| + | CHMT-2, 1 (1979) 70-75 |
| − | the extrusion direction ''(Figs. 2.130 – 2.133)''. Depending on the extrusion
| |
| − | method in either rod or strip form the graphite particles can be oriented in the
| |
| − | finished contact tips perpendicular (GRAPHOR) or parallel (GRAPHOR D) to the
| |
| − | switching contact surface ''(Figs. 2.131 and 2.132)''.
| |
| | | | |
| − | Since the graphite particles in the Ag matrix of Ag/C materials prevent contact
| + | Leung, C.-H.; Kim, H.J.: A Comparison of Ag/W, Ag/WC and Ag/Mo Electrical |
| − | tips from directly being welded or brazed, a graphite free bottom layer is
| + | Contacts. IEEE Trans. Components, Hybrids, Manuf. Technol., |
| − | required. This is achieved by either burning out (de-graphitizing) the graphite
| + | Vol. CHMT-7, 1 (1984) 69-75 |
| − | selectively on one side of the tips or by compound extrusion of a Ag/C billet
| |
| − | covered with a fine silver shell.
| |
| | | | |
| − | Ag/C contact materials exhibit on the one hand an extremely high resistance to
| + | Allen, S.E.; Streicher, E.: The Effect of Microstructure on the Electrical |
| − | contact welding but on the other have a low arc erosion resistance. This is
| + | th Performance of Ag-WC-C Contact Materials. Proc. 44 IEEE Holm Conf. on Electr. |
| − | caused by the reaction of graphite with the oxygen in the surrounding
| + | Contacts, Arlington, VA, USA (1998), 276-285 |
| − | atmosphere at the high temperatures created by the arcing. The weld resistance
| |
| − | is especially high for materials with the graphite particle orientation parallel to the
| |
| − | arcing contact surface. Since the contact surface after arcing consists of pure
| |
| − | silver the contact resistance stays consistently low during the electrical life of the
| |
| − | contact parts.
| |
| | | | |
| − | A disadvantage of the Ag/C materials is their rather high erosion rate. In materials
| + | Haufe, W.; Reichel, W.; Schreiner H.: Abbrand verschiedener W/Cu-Sinter- |
| − | with parallel graphite orientation this can be improved if part of the graphite is
| + | Tränkwerkstoffe an Luft bei hohen Strömen. Z. Metallkd. 63 (1972) 651-654 |
| − | incorporated into the material in the form of fibers (GRAPHOR DF), ''(Fig. 2.133)''.
| |
| − | The weld resistance is determined by the total content of graphite particles.
| |
| | | | |
| − | Ag/C tips with vertical graphite particle orientation are produced in a specific
| + | Althaus, B.; Vinaricky, E.: Das Abbrandverhalten verschieden hergestellter |
| − | sequence: Extrusion to rods, cutting of double thickness tips, burning out of
| + | Wolfram-Kupfer-Verbundwerkstoffe im Hochstromlichtbogen. |
| − | graphite to a controlled layer thickness, and a second cutting to single tips.
| + | Metall 22 (1968) 697-701 |
| − | Such contact tips are especially well suited for applications which require both,
| |
| − | a high weld resistance and a sufficiently high arc erosion resistance ''(Table 2.33)''.
| |
| − | For attachment of Ag/C tips welding and brazing techniques are applied.
| |
| | | | |
| − | welding the actual process depends on the material's graphite orientation. For
| + | Gessinger, G.H.; Melton, K.N.: Burn-off Behaviour of WCu Contact Materials in an |
| − | Ag/C tips with vertical graphite orientation the contacts are assembled with
| + | Electric Arc. Powder Metall. Int. 9 (1977) 67-72 |
| − | single tips. For parallel orientation a more economical attachment starting with
| |
| − | contact material in strip or profile tape form is used in integrated stamping and
| |
| − | welding operations with the tape fed into the weld station, cut off to tip form and
| |
| − | then welded to the carrier material before forming the final contact assembly
| |
| − | part. For special low energy welding the Ag/C profile tapes GRAPHOR D and DF
| |
| − | can be pre-coated with a thin layer of high temperature brazing alloys such as
| |
| − | CuAgP.
| |
| | | | |
| − | In a rather limited way, Ag/C with 2 – 3 wt% graphite can be produced in wire
| + | Magnusson, M.: Abbrandverhalten und Rißbildung bei WCu-Tränkwerkstoffen |
| − | form and headed into contact rivet shape with low head deformation ratios.
| + | unterschiedlicher Wolframteilchengröße. ETZ-A 98 (1977) 681-683 |
| | | | |
| − | The main applications for Ag/C materials are protective switching devices such
| + | Heitzinger, F.; Kippenberg, H.; Saeger, K.E.; Schröder, K.H.: Contact Materials for |
| − | as miniature molded case circuit breakers, motor-protective circuit breakers,
| + | Vacuum Switching Devices. Proc. XVth ISDEIV, Darmstadt 1992, 273-278 |
| − | and fault current circuit breakers, where during short circuit failures highest
| |
| − | resistance against welding is required ''(Table 2.34)''. For higher currents the low
| |
| − | arc erosion resistance of Ag/C is compensated by asymmetrical pairing with
| |
| − | more erosion resistant materials such as Ag/Ni and Ag/W.
| |
| | | | |
| − | Fig. 2.126:
| + | Grill, R.; Müller, F.: Verbundwerkstoffe auf Wolframbasis für |
| − | Strain hardening
| + | Hochspannungsschaltgeräte. Metall 61 (2007) H. 6, 390-393 |
| − | of Ag/C 96/4 D
| |
| − | by cold working
| |
| | | | |
| − | Fig. 2.127:
| + | Slade, P.: G.: The Vacuum Interrupter- Theory; Design; and Application. CRC |
| − | Softening of Ag/C 96/4 D after
| + | Press, Boca Raton, FL (USA), 2008 |
| − | annealing
| |
| | | | |
| − | Fig. 2.128: Strain hardening
| + | Frey, P.; Klink, N.; Saeger, K.E.: Untersuchungen zum Abreißstromverhalten von |
| − | of Ag/C DF by cold working
| + | Kontaktwerkstoffen für Vakuumschütze. Metall 38 (1984) 647-651 |
| | | | |
| − | Fig. 2.129: Softening
| + | Frey, P.; Klink, N.; Michal, R.; Saeger, K.E.: Metallurgical Aspects of Contact |
| − | of Ag/C DF after annealing
| + | Materials for Vacuum Switching Devices. IEEE Trans. Plasma Sc. 17, (1989) 743- |
| | + | 740 |
| | | | |
| − | Fig. 2.130: Micro structure of Ag/C 97/3: a) perpendicular to extrusion direction
| + | Slade, P.: Advances in Material Development for High Power Vacuum Interrupter |
| − | b) parallel to extrusion direction, 1) Ag/C contact layer, 2) Ag backing layer
| + | th Contacts. Proc.16 Int. Conf. on Electr. Contact Phenom., |
| | + | Loughborough 1992,1-10 |
| | | | |
| − | Fig. 2.131: Micro structure of Ag/C 95/5: a) perpendicular to extrusion direction
| + | Behrens, V.; Honig, Th.; Kraus, A.; Allen, S.: Comparison of Different Contact |
| − | b) parallel to extrusion direction, 1) Ag/C contact layer, 2) Ag backing layer
| + | th Materials for Low Voltage Vacuum Applications. Proc.19 Int. Conf. on Electr. |
| | + | Contact Phenom., Nuremberg 1998, 247-251 |
| | | | |
| − | Fig. 2.132: Micro structure of Ag/C 96/4 D: a) perpendicular to extrusion direction
| + | Rolle, S.; Lietz, A.; Amft, D.; Hauner, F.: CuCr Contact Material for Low Voltage |
| − | b) parallel to extrusion direction, 1) Ag/C contact layer, 2) Ag backing layer
| + | th Vacuum Contactors. Proc. 20 int. Conf. on Electr. Contact. Phenom. Stockholm |
| | + | 2000, 179-186 |
| | | | |
| − | Fig. 2.133: Micro structure of Ag/C DF: a) perpendicular to extrusion direction
| + | Kippenberg, H.: CrCu as a Contact Material for Vacuum Interrupters. |
| − | b) parallel to extrusion direction, 1) Ag/C contact layer, 2) Ag/Ni 90/10 backing layer
| + | th Proc.13 Int. Conf. on Electr. Contact Phenom. Lausanne 1986, 140-144 |
| | | | |
| − | Table 2.32: Physical Properties of Silver–Graphite (GRAPHOR) Contact Materials
| + | Hauner, F.; Müller, R.; Tiefel, R.: CuCr für Vakuumschaltgeräte- |
| | + | Herstellungsverfahren, Eigenschaften und Anwendung. |
| | + | Metall 61 (2007) H. 6, 385-389 |
| | | | |
| − | Table 2.33: Contact and Switching properties of Silver–Graphite (GRAPHOR) Contact Materials
| + | Manufacturing Equipment for Semi-Finished Materials |
| | + | (Bild) |
| | | | |
| − | Table 2.34: Application Examples and Forms of Supply of Silver–
| + | [[de:Kontaktwerkstoffe_für_die_Elektrotechnik]] |
| − | Graphite (GRAPHOR) Contact Materials
| |
The contact parts are important components in switching devices. They have to maintain their function from the new state until the end of the functional life of the devices.
The requirements on contacts are rather broad. Besides typical contact properties such as
- High arc erosion resistance
- High resistance against welding
- Low contact resistance
- Good arc moving properties
- Good arc extinguishing capability
They have to exhibit physical, mechanical and chemical properties like high electrical and thermal conductivity, high hardness, high corrosion resistance etc. and besides this, should have good mechanical workability and also be suitable for good weld and brazing attachment to contact carriers. In addition they must be made from environmentally friendly materials.
Materials suited for use as electrical contacts can be divided into the following groups based on their composition and metallurgical structure:
- Pure metals
- Alloys
- Composite materials
Pure metals
Within this group, silver has the greatest importance for switching devices in the higher energy technology. Other precious metals such as gold and platinum are only used in applications for the information technology in the form of thin surface layers. As a nonprecious metal, tungsten is used for some special applications such as, for example, automotive horn contacts. In some rarer cases, pure copper is used, but mainly paired to a silver-based contact material.
Alloys
Besides these few pure metals, a larger number of alloy materials made by melt technology are available for the use as contacts. An alloy is characterized by the fact, that its components are completely or partially soluble in each other in the solid state. Phase diagrams for multiple metal compositions show the number and type of the crystal structure as a function of the temperature and composition of the alloying components.
They indicate the boundaries of liquid and solid phases and define the parameters of solidification.
Alloying allows to improve the properties of one material at the cost of changing them for the second material. As an example, the hardness of a base metal may be increased while at the same time the electrical conductivity decreases with even small additions of the second alloying component.
Composite Materials
Composite materials are a material group whose properties are of great importance for electrical contacts that are used in switching devices for higher
electrical currents.
Those used in electrical contacts are heterogeneous materials, composed of two or more uniformly dispersed components, in which the largest volume portion consists of a metal.
The properties of composite materials are determined mainly independent from each other by the properties of their individual components. Therefore it is, for example, possible to combine the high melting point and arc erosion resistance of tungsten with the low melting and good electrical conductivity of copper or the high conductivity of silver with the weld resistant metalloid graphite. Figure 1 shows the schematic manufacturing processes from powder blending to contact material. Three basic process variations are typically applied:
- Sintering without liquid phase (Press-Sinter-Repress, PSR)
- Sintering with liquid phase
- Infiltration (Press-Sinter-Infiltrate, PSI)
During sintering without a liquid phase (left side of schematic), the powder mix is first densified by pressing, then undergoes a heat treatment (sintering) and eventually is re-pressed again to further increase the density. The sintering atmosphere depends on the material components and later application; a vacuum is used for example for the low gas content material Cu/Cr. This process is used for individual contact parts and also termed press-sinter-repress (PSR). For materials with high silver content, the starting point before pressing is mostly a large block (or billet) which is then, after sintering, hot extruded into wire, rod or strip form. The extrusion further increases the density of these composite materials and contributes to higher arc erosion resistance. Materials such as Ag/Ni, Ag/MeO and Ag/C are typically produced by this process.
Sintering with liquid phase has the advantage of shorter process times due to the accelerated diffusion and also results in near-theoretical densities of the composite material. To ensure the shape stability during the sintering process, it
is however necessary to limit the volume content of the liquid phase material.
As opposed to the liquid phase sintering, which has limited use for electrical contact manufacturing, the Infiltration process as shown on the right side of the schematic, has a broad practical range of applications. In this process the powder of the higher melting component, sometimes also as a powder mix with a small amount of the second material, is pressed into parts. Then, right after sintering, the porous skeleton is infiltrated with liquid metal of the second material. The fill-up process of the pores happens through capillary forces. This process reaches, after the infiltration, near-theoretical density without subsequent pressing and is widely used for Ag- and Cu-refractory contacts. For Ag/W or Ag/WC contacts, controlling the amount or excess on the bottom side of the contact of the infiltration metal Ag, results in contact tips that can be easily attached to their carriers by resistance welding. For larger Cu/W contacts, additional machining is often used to obtain the final shape of the contact component.
Gold Based Materials
Pure Gold is besides Platinum the chemically most stable of all precious metals. In its pure form, it is not very suitable for use as a contact material in electromechanical devices because of its tendency to stick and cold-weld at even low contact forces. In addition, it is not hard or strong enough to resist mechanical wear and exhibits high material losses under electrical arcing loads. This limits its use in form of thin electroplated or vacuum deposited layers.
Main Article: Gold Based Materials
Platinum Metal Based Materials
The platinum group metals include the elements Pt, Pd, Rh, Ru, Ir and Os (Table 1). For electrical contacts, platinum and palladium have practical significance as base alloy materials and ruthenium and iridium are used as alloying components. Pt and Pd have similar corrosion resistance as gold but due to their catalytical properties, they tend to polymerize adsorbed organic vapors on contact surfaces. During frictional movement between contact surfaces, the polymerized compounds known as “brown powder” are formed, which can lead to a significant increase in contact resistance. Therefore Pt and Pd are typically used as alloys and are rather not used in their pure form for electrical contact applications.
Main Article: Platinum Metal Based Materials
Silver Based Materials
Main Article: Silver Based Materials
Tungsten and Molybdenum Based Materials
Main Article: Tungsten and Molybdenum Based Materials
Contact Materials for Vacuum Switches
The low gas content contact materials are developed for the use in vacuum switching devices.
Main Article: Contact Materials for Vacuum Switches
References
Vinaricky, E.(Hrsg.): Elektrische Kontakte, Werkstoffe und Anwendungen.
Springer-Verlag, Berlin, Heidelberg etc. 2002
Lindmayer, M.: Schaltgeräte-Grundlagen, Aufbau, Wirkungsweise.
Springer-Verlag, Berlin, Heidelberg, New York, Tokio, 1987
Rau, G.: Metallische Verbundwerkstoffe. Werkstofftechnische
Verlagsgesellschaft, Karlsruhe 1977
Schreiner, H.: Pulvermetallurgie elektrischer Kontakte. Springer-Verlag
Berlin, Göttingen, Heidelberg, 1964
Hansen. M.; Anderko, K.: Constitution of Binary Alloys. New York:
Mc Graw-Hill, 1958
Shunk, F.A.: Constitution of Binary Alloy. 2 Suppl. New York; Mc Graw-Hill, 1969
Edelmetall-Taschenbuch. ( Herausgeber Degussa AG, Frankfurt a. M.),
Heidelberg, Hüthig-Verlag, 1995
Rau, G.: Elektrische Kontakte-Werkstoffe und Technologie. Eigenverlag G. Rau
GmbH & Co., Pforzheim, 1984
Heraeus, W. C.: Werkstoffdaten. Eigenverlag W.C. Heraeus, Hanau, 1978
Linde, J.O.: Elektrische Widerstandseigenschaften der verdünnten Legierungen
des Kupfers, Silbers und Goldes. Lund: Hakan Ohlsson, 1938
Engineers Relay Handbook, RSIA, 2006
Großmann, H. Saeger, K. E.; Vinaricky, E.: Gold and Gold Alloys in Electrical
Engineering. in: Gold, Progress in Chemistry, Biochemistry and Technology. John
Wiley & Sons, Chichester etc, (1999) 199-236
Gehlert, B.: Edelmetall-Legierungen für elektrische Kontakte.
Metall 61 (2007) H. 6, 374-379
Aldinger, F.; Schnabl, R.: Edelmetallarme Kontakte für kleine Ströme.
Metall 37 (1983) 23-29
Bischoff, A.; Aldinger, F.: Einfluss geringer Zusätze auf die mechanischen
Eigenschaften von Au-Ag-Pd-Legierungen. Metall 36 (1982) 752-765
Wise, E.M.: Palladium, Recovery, Properties and Uses. New York, London:
Academic Press 1968
Savitskii, E.M.; Polyakova, V.P.; Tylina, M.A.: Palladium Alloys, Primary Sources.
New York: Publishers 1969
Gehlert, B.: Lebensdaueruntersuchungen von Edelmetall Kontaktwerkstoff-
Kombinationen für Schleifringübertrager. VDE-Fachbericht 61, (2005) 95-100
Holzapfel,C.: Verschweiß und elektrische Eigenschaften von
Schleifringübertragern. VDE-Fachbericht 67 (2011) 111-120
Schnabl, R.; Gehlert, B.: Lebensdauerprüfungen von Edelmetall-
Schleifkontaktwerkstoffen für Gleichstrom Kleinmotoren.
Feinwerktechnik & Messtechnik (1984) 8, 389-393
Kobayashi, T.; Koibuchi, K.; Sawa, K.; Endo, K.; Hagino, H.: A Study of Lifetime
of Au-plated Slip-Ring and AgPd Brush System for Power Supply.
th Proc. 24 Int. Conf. on Electr. Contacts, Saint Malo, France 2008, 537-542
Harmsen, U.; Saeger K.E.: Über das Entfestigungsverhalten von Silber
verschiedener Reinheiten. Metall 28 (1974) 683-686
Behrens, V.; Michal, R.; Minkenberg, J.N.; Saeger, K.E.: Abbrand und
Kontaktwiderstandsverhalten von Kontaktwerkstoffen auf Basis von Silber-
Nickel. e.& i. 107. Jg. (1990), 2, 72-77
Behrens, V.: Silber/Nickel und Silber/Grafit- zwei Spezialisten auf dem Gebiet
der Kontaktwerkstoffe. Metall 61 (2007) H.6, 380-384
Rieder, W.: Silber / Metalloxyd-Werkstoffe für elektrische Kontakte,
VDE - Fachbericht 42 (1991) 65-81
Harmsen,U.: Die innere Oxidation von AgCd-Legierungen unter
Sauerstoffdruck.
Metall 25 (1991), H.2, 133-137
Muravjeva, E.M.; Povoloskaja, M.D.: Verbundwerkstoffe Silber-Zinkoxid und
Silber-Zinnoxid, hergestellt durch Oxidationsglühen.
Elektrotechnika 3 (1965) 37-39
Behrens, V.; Honig Th.; Kraus, A.; Michal, R.; Saeger, K.-E.; Schmidberger, R.;
Staneff, Th.: Eine neue Generation von AgSnO2 -Kontaktwerkstoffen.
VDE-Fachbericht 44, (1993) 99-114
Braumann, P.; Lang, J.: Kontaktverhalten von Ag-Metalloxiden für den Bereich
hoher Ströme. VDE-Fachbericht 42, (1991) 89-94
Hauner, F.; Jeannot, D.; Mc Neilly, U.; Pinard, J.: Advanced AgSnO Contact 2
th Materials for High Current Contactors. Proc. 20 Int. Conf. on Electr. Contact
Phenom., Stockholm 2000, 193-198
Wintz, J.-L.; Hardy, S.; Bourda, C.: Influence on the Electrical Performances of
Assembly Process, Supports Materials and Production Means for AgSnO2 .
Proc.24th Int. Conf. on Electr. Contacts, Saint Malo, France 2008, 75-81
Behrens, V.; Honig, Th.; Kraus, A.; Michal, R.: Schalteigenschaften von
verschiedenen Silber-Zinnoxidwerkstoffen in Kfz-Relais. VDE-Fachbericht 51
(1997) 51-57
Schöpf, Th.: Silber/Zinnoxid und andere Silber-Metalloxidwerkstoffe in
Netzrelais. VDE-Fachbericht 51 (1997) 41-50
Schöpf, Th.; Behrens, V.; Honig, Th.; Kraus, A.: Development of Silver Zinc
th Oxide for General-Purpose Relays. Proc. 20 Int. Conf. on Electr. Contacts,
Stockholm 2000, 187-192
Braumann, P.; Koffler, A.: Einfluss von Herstellverfahren, Metalloxidgehalt und
Wirkzusätzen auf das Schaltverhalten von Ag/SnO in Relais. 2
VDE-Fachbericht 59, (2003) 133-142
Kempf, B.; Braumann, P.; Böhm, C.; Fischer-Bühner, J.: Silber-Zinnoxid-
Werkstoffe: Herstellverfahren und Eigenschaften. Metall 61(2007) H. 6, 404-408
Lutz, O.; Behrens, V.; Finkbeiner, M.; Honig, T.; Späth, D.: Ag/CdO-Ersatz in
Lichtschaltern. VDE-Fachbericht 61, (2005) 165-173
Lutz, O.; Behrens, V.; Wasserbäch, W.; Franz, S.; Honig, Th.; Späth,
D.; Heinrich, J.: Improved Silver/Tin Oxide Contact Materials for Automotive
th Applications. Proc.24 Int. Conf. on Electr. Contacts, Saint Malo, France 2008,
88-93
Leung, C.; Behrens, V.: A Review of Ag/SnO Contact Materials and Arc Erosion. 2
th Proc.24 Int. Conf. on Electr. Contacts, Saint Malo, France 2008, 82-87
Chen, Z.K.; Witter, G.J.: Comparison in Performance for Silver–Tin–Indium
Oxide Materials Made by Internal Oxidation and Powder Metallurgy.
th Proc. 55 IEEE Holm Conf. on Electrical Contacts, Vancouver, BC, Canada,
(2009) 167 – 176
Roehberg, J.; Honig, Th.; Witulski, N.; Finkbeiner, M.; Behrens, V.: Performance
of Different Silver/Tin Oxide Contact Materials for Applications in Low Voltage
th Circuit Breakers. Proc. 55 IEEE Holm Conf. on Electrical Contacts, Vancouver,
BC, Canada, (2009) 187 – 194
Muetzel, T.; Braumann, P.; Niederreuther, R.: Temperature Rise Behavior of
th Ag/SnO Contact Materials for Contactor Applications. Proc. 55 IEEE Holm 2
Conf. on Electrical Contacts, Vancouver, BC, Canada, (2009) 200 – 205
Lutz, O. et al.: Silber/Zinnoxid – Kontaktwerkstoffe auf Basis der Inneren
Oxidation fuer AC – und DC – Anwendungen.
VDE Fachbericht 65 (2009) 167 – 176
Harmsen, U.; Meyer, C.L.: Mechanische Eigenschaften stranggepresster Silber-
Graphit-Verbundwerkstoffe. Metall 21 (1967), 731-733
Behrens, V.: Mahle, E.; Michal, R.; Saeger, K.E.: An Advanced Silver/Graphite
th Contact Material Based on Graphite Fibre. Proc. 16 Int. Conf. on Electr.
Contacts, Loghborough 1992, 185-189
Schröder, K.-H.; Schulz, E.-D.: Über den Einfluss des Herstellungsverfahrens
th auf das Schaltverhalten von Kontaktwerkstoffen der Energietechnik. Proc. 7 Int.
Conf. on Electr. Contacts, Paris 1974, 38-45
Mützel, T.: Niederreuther, R.: Kontaktwerkstoffe für Hochleistungsanwendungen.
VDE-Bericht 67 (2011) 103-110
Lambert, C.; Cambon, G.: The Influence of Manufacturing Conditions and
Metalurgical Characteristics on the Electrical Behaviour of Silver-Graphite
th Contact Materials. Proc. 9 Int. Conf.on Electr. Contacts,
Chicago 1978, 401-406
Vinaricky, E.: Grundsätzliche Untersuchungen zum Abbrand- und
Schweißverhalten von Ag/C-Kontaktwerkstoffen. VDE-Fachbericht 47 (1995)
159-169
Agte, C.; Vacek, J.: Wolfram und Molybdän. Berlin: Akademie-Verlag 1959
Keil, A.; Meyer, C.-L.: Der Einfluß des Faserverlaufes auf die elektrische
Verschleißfestigkeit von Wolfram-Kontakten. ETZ 72, (1951) 343-346
Slade, P. G.: Electric Contacts for Power Interruption. A Review. Proc. 19 Int.
Conf. on Electric Contact Phenom. Nuremberg (Germany) 1998, 239-245
Slade, P. G.: Variations in Contact Resistance Resulting from Oxide Formation
and Decomposition in AgW and Ag-WC-C Contacts Passing Steady Currents
for Long Time Periods. IEEE Trans. Components, Hybrids and Manuf. Technol.
CHMT-9,1 (1986) 3-16
Slade, P. G.: Effect of the Electric Arc and the Ambient Air on the Contact
Resistance of Silver, Tungsten and Silver-Tungsten Contacts.
J.Appl.Phys. 47, 8 (1976) 3438-3443
Lindmayer, M.; Roth, M.: Contact Resistance and Arc-Erosion of W-Ag and
WC-Ag. IEEE Trans components, Hybrids and Manuf. Technol.
CHMT-2, 1 (1979) 70-75
Leung, C.-H.; Kim, H.J.: A Comparison of Ag/W, Ag/WC and Ag/Mo Electrical
Contacts. IEEE Trans. Components, Hybrids, Manuf. Technol.,
Vol. CHMT-7, 1 (1984) 69-75
Allen, S.E.; Streicher, E.: The Effect of Microstructure on the Electrical
th Performance of Ag-WC-C Contact Materials. Proc. 44 IEEE Holm Conf. on Electr.
Contacts, Arlington, VA, USA (1998), 276-285
Haufe, W.; Reichel, W.; Schreiner H.: Abbrand verschiedener W/Cu-Sinter-
Tränkwerkstoffe an Luft bei hohen Strömen. Z. Metallkd. 63 (1972) 651-654
Althaus, B.; Vinaricky, E.: Das Abbrandverhalten verschieden hergestellter
Wolfram-Kupfer-Verbundwerkstoffe im Hochstromlichtbogen.
Metall 22 (1968) 697-701
Gessinger, G.H.; Melton, K.N.: Burn-off Behaviour of WCu Contact Materials in an
Electric Arc. Powder Metall. Int. 9 (1977) 67-72
Magnusson, M.: Abbrandverhalten und Rißbildung bei WCu-Tränkwerkstoffen
unterschiedlicher Wolframteilchengröße. ETZ-A 98 (1977) 681-683
Heitzinger, F.; Kippenberg, H.; Saeger, K.E.; Schröder, K.H.: Contact Materials for
Vacuum Switching Devices. Proc. XVth ISDEIV, Darmstadt 1992, 273-278
Grill, R.; Müller, F.: Verbundwerkstoffe auf Wolframbasis für
Hochspannungsschaltgeräte. Metall 61 (2007) H. 6, 390-393
Slade, P.: G.: The Vacuum Interrupter- Theory; Design; and Application. CRC
Press, Boca Raton, FL (USA), 2008
Frey, P.; Klink, N.; Saeger, K.E.: Untersuchungen zum Abreißstromverhalten von
Kontaktwerkstoffen für Vakuumschütze. Metall 38 (1984) 647-651
Frey, P.; Klink, N.; Michal, R.; Saeger, K.E.: Metallurgical Aspects of Contact
Materials for Vacuum Switching Devices. IEEE Trans. Plasma Sc. 17, (1989) 743-
740
Slade, P.: Advances in Material Development for High Power Vacuum Interrupter
th Contacts. Proc.16 Int. Conf. on Electr. Contact Phenom.,
Loughborough 1992,1-10
Behrens, V.; Honig, Th.; Kraus, A.; Allen, S.: Comparison of Different Contact
th Materials for Low Voltage Vacuum Applications. Proc.19 Int. Conf. on Electr.
Contact Phenom., Nuremberg 1998, 247-251
Rolle, S.; Lietz, A.; Amft, D.; Hauner, F.: CuCr Contact Material for Low Voltage
th Vacuum Contactors. Proc. 20 int. Conf. on Electr. Contact. Phenom. Stockholm
2000, 179-186
Kippenberg, H.: CrCu as a Contact Material for Vacuum Interrupters.
th Proc.13 Int. Conf. on Electr. Contact Phenom. Lausanne 1986, 140-144
Hauner, F.; Müller, R.; Tiefel, R.: CuCr für Vakuumschaltgeräte-
Herstellungsverfahren, Eigenschaften und Anwendung.
Metall 61 (2007) H. 6, 385-389
Manufacturing Equipment for Semi-Finished Materials
(Bild)