Contact Carrier Materials
The reliability and electrical life of contact systems in switching devices as well as in electromechanical and electronic components do not only depend on the contact material. The selection of the most suitable carrier material also plays an important role.
The most frequently used ones are copper based materials. Depending on the application also materials based on nickel or multi-layer composite materials, such as thermo bimetals for example, are frequently used. For special applications in the medium and high voltage technology, as well as for springs and snap discs for the information technology, iron or steel based materials are considered. These are however not included for the purpose of this data book.
Various requirements based on the enduse of the contact components have to be met by carrier materials. Copper materials have to exhibit high electrical and thermal conductivity, good mechanical strength even at elevated temperatures, and in addition a sufficient high resistance against corrosion. If used as springs the carrier materials also must have good elastic spring properties. Besides these, the materials must, depending on the manufacturing processes employed, also have good technological properties like ductility to allow warm and cold forming, suitability for cutting and stamping, and be capable to be welded, brazed or coated by electroplating
Contents
5.1 Copper and Copper Alloys
5.1.1 Standards Overview
Copper and copper alloys to be used in electrical and electronic components are usually covered by national and international standards. DIN numbers the materials by a prefix and/or a material number. The newer European standards (EN) refer to the material's usage products and also show a prefix and material number. For reference we also show in table 5.1 the material designation according to UNS, the Unified Numbering System (USA). Other internationally used standard and material numbers include, among others, those issued by CDA (Copper Development Association, USA), and GB (Guo Biao – China).
The most important EN as well as the US based and widely used ASTM standards covering the use of flat rolled copper and copper alloys in electrical contacts are:
| Standard Designation | D e s c ription |
|---|---|
| DIN EN 1652 | Copper and copper alloys in plate, sheet, strip, and discs for general applications |
| DIN EN 1654 | Copper and copper alloys for springs and connectors |
| DIN EN 1758 | Copper and copper alloys in strip form for system component carriers |
| ASTM B 103/B103M-10 | Spec. for Phosphor Bronce Plate, Sheet, Strip, and Rolled Bar |
| ASTM B 36/B36M-95 | Spec. for Brass Plate, Sheet, Strip, and Rolled Bar |
| ASTM B 122/B122M-08 | Spec. for CuNiSn-, CuNiZn-, and CuNi-Alloy |
| ASTM B 465-09 | Spec. for Copper-Iron-Alloy Plate, Sheet, and Strip |
| ASTM B 194-08 | Standard Spec. for CuBe-Alloy Plate, Sheet, Strip and Rolled Bar |
| ASTM B 534-07 | Sec. for CuCoBe-Alloy and CuNiBe-Alloy Plate, Sheet, Strip, and Rolled Bar |
The above DIN EN standards replace in part or completely the older DIN standards DIN 1777, DIN 17670, DIN 1751, DIN 1791.
5.1.2 Pure Copper
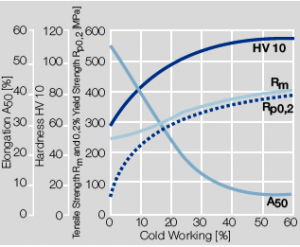
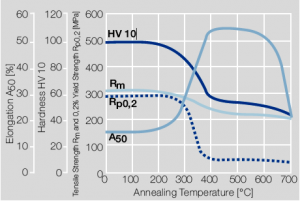
Copper is used in electrical engineering mostly because of its high electrical conductivity which with 58 MS/m (or m/Ωmm²) is only slightly below that of silver. Other advantages of copper are its high thermal conductivity, corrosion resistance, and its good ductility. The work hardening properties of ETP copper is illustrated in Figure 1. The increase in strength achieved by cold working can be reversed easily by subsequent annealing. The softening properties are strongly dependent on the preceding cold working percentage (Figure 2 and 5.3).
The purity of technically pure and un-alloyed copper used for electrical applications depends on the type used and ranges between > 99.90 and 99.95 wt%. The copper types are designated mainly by their oxygen content as oxygen containing, oxygen-free, and de-oxidized with phosphorus as described in DIN EN 1652 (Table 1 and 5.2).Tables 5.3. and 5.4 show the physical and mechanical properties of these copper materials. According to these, Cu-ETP, Cu-OFE, and Cu-HCP are the types of copper for which minimum values for the electrical conductivity are guaranteed. Cu-ETP is less suitable for welding or for brazing in reducing atmosphere because of the oxygen content (danger of hydrogen embrittlement). Cu-HCP, Cu-DLP, and Cu-DHP are oxygen free copper types de-oxidized with different phosphorus contents. With increasing phosphorus content the electrical conductivity decreases. Cu-OFE, also called OFHC copper, is free of oxygen and also free of de-oxidizing compounds.
- ) As units for electrical conductivity MS/m and m/Ω.mm² are commonly used. Frequently – and
mostly in North America – the % IACS value (International Annealed Copper Standard) is also used, 2 where 100% is equivalent to 58 MS/m or m/Ωmm .For the description of mechanical strength 2 properties the units of N/mm or MPa are most commonly used: 2 1 MS/m = 1 m/Ωmm 2 1 MPa = 1 N/mm 5.1.2 Pure Copper
| ASTM B 534-07 | Sec. for CuCoBe-Alloy and CuNiBe-Alloy Plate, Sheet, Strip, and Rolled Bar |
Tab 5.2
Table 5.3: Physical Properties of Some Copper Types
Table 5.4: Mechanical Properties of Some Copper Types
Fig. 5.1: Strain hardening of Cu-ETP by cold working
Fig. 5.2: Softening of Cu-ETP after annealing for 3hrs after 25% cold working
Fig. 5.3: Softening of Cu-ETP after annealing for 3hrs after 50% cold working
5.1.3 High Cu Content Copper Alloys
The high Cu content alloy materials are closest in their properties to pure copper materials. By defined addition of small amounts of alloying elements it is possible to increase the mechanical strength and especially the softening temperature of copper and at the same time decrease the electrical conductivity only insignificantly (Fig. 5.4). Silver, iron, tin, zinc, nickel, chromium, zirconium, silicon, and titanium are used. Usually the additive amounts are significantly below 3 wt%. This group of materials consists of mixed crystal as well as precipitation hardening alloys. The precipiytion hardening copper-beryllium and copper-chromium-zirconium materials are decribed later in a separate section.