Difference between revisions of "Physikalische Eigenschaften der wichtigsten Metalle"
Doduco Admin (talk | contribs) |
Doduco Admin (talk | contribs) |
||
| Line 641: | Line 641: | ||
|23,6 | |23,6 | ||
| -6,5 | | -6,5 | ||
| + | |- | ||
| + | |Antimon | ||
| + | |0,210 | ||
| + | | | ||
| + | |630 | ||
| + | |163 | ||
| + | |2,5x10<sup>-9</sup> | ||
| + | |1587 | ||
| + | |1,97 | ||
| + | |24,3 | ||
| + | |10,5 | ||
| + | | +9,5 | ||
| + | |- | ||
| + | |Beryllium | ||
| + | |1,824 | ||
| + | | | ||
| + | |1277 | ||
| + | |1090 | ||
| + | |4,3 | ||
| + | |2477 | ||
| + | | | ||
| + | |200 | ||
| + | |12,3 | ||
| + | | | ||
| + | |- | ||
| + | |Blei | ||
| + | |0,130 | ||
| + | |200 | ||
| + | |327 | ||
| + | |25 | ||
| + | |4,21x10<sup>-7</sup> | ||
| + | |1750 | ||
| + | |24,70 | ||
| + | |35,3 | ||
| + | |29,3 | ||
| + | | -3,5 | ||
| + | |- | ||
| + | |Cadmium | ||
| + | |0,230 | ||
| + | | | ||
| + | |321 | ||
| + | |54 | ||
| + | |14,8 | ||
| + | |767 | ||
| + | |0,88 | ||
| + | |96,8 | ||
| + | |41,0 | ||
| + | | -4,0 | ||
| + | |- | ||
| + | |Chrom | ||
| + | |0,450 | ||
| + | | | ||
| + | |1857 | ||
| + | |314 | ||
| + | |990 | ||
| + | |2672 | ||
| + | |5,86 | ||
| + | |93,7 | ||
| + | |6,2 | ||
| + | | | ||
| + | |- | ||
| + | |Eisen | ||
| + | |0,444 | ||
| + | |500 | ||
| + | |1537 | ||
| + | |268 | ||
| + | |7,05 | ||
| + | |2750 | ||
| + | |80,2 | ||
| + | |12,2 | ||
| + | | -3,0 | ||
| + | | | ||
| + | |- | ||
| + | |Gallium | ||
| + | |0,370 | ||
| + | | | ||
| + | |29,8 | ||
| + | |80,4 | ||
| + | |9,6x10<sup>-36</sup> | ||
| + | |2204 | ||
| + | |3,90 | ||
| + | |40,6 | ||
| + | |18,0 | ||
| + | | +3,0 | ||
| + | |- | ||
| + | |Gold | ||
| + | |0,128 | ||
| + | |100 | ||
| + | |1064 | ||
| + | |63 | ||
| + | |2,4x10<sup>-3</sup> | ||
| + | |3080 | ||
| + | |1,55 | ||
| + | |317 | ||
| + | |14,3 | ||
| + | | -5,1 | ||
| + | |- | ||
| + | |Indium | ||
| + | |0,233 | ||
| + | | | ||
| + | |157 | ||
| + | |28,5 | ||
| + | |1,5x10<sup>-17</sup> | ||
| + | |2072 | ||
| + | |1,97 | ||
| + | |81,6 | ||
| + | | | ||
| + | | -2,5 | ||
| + | |- | ||
| + | |Iridium | ||
| + | |0,130 | ||
| + | | | ||
| + | |2410 | ||
| + | |144 | ||
| + | |1,5 | ||
| + | |4130 | ||
| + | |3,31 | ||
| + | |147 | ||
| + | |6,5 | ||
| + | | | ||
| + | |- | ||
| + | |Cobalt | ||
| + | |0,420 | ||
| + | | | ||
| + | |1495 | ||
| + | |260 | ||
| + | |175 | ||
| + | |2927 | ||
| + | |6,66 | ||
| + | |100 | ||
| + | |13,8 | ||
| + | | | ||
| + | |- | ||
| + | |Kohlenstoff (Graphit) | ||
| + | |0,720 | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |3825 sublimiert | ||
| + | |119 - 165 | ||
| + | |155 | ||
| + | | | ||
| + | | | ||
| + | |- | ||
| + | |Kupfer | ||
| + | |0,385 | ||
| + | |190 | ||
| + | |1084 | ||
| + | |205 | ||
| + | |5,2x10<sup>-2</sup> | ||
| + | |2567 | ||
| + | |4,77 | ||
| + | |401 | ||
| + | |16,5 | ||
| + | | -4,2 | ||
| + | |- | ||
| + | |Magnesium | ||
| + | |1,020 | ||
| + | | | ||
| + | |650 | ||
| + | |373 | ||
| + | |361 | ||
| + | |1107 | ||
| + | |5,44 | ||
| + | |156 | ||
| + | |26,0 | ||
| + | | -4,1 | ||
| + | |- | ||
| + | |Mangan | ||
| + | |0,480 | ||
| + | | | ||
| + | |1244 | ||
| + | |264 | ||
| + | |121 | ||
| + | |1962 | ||
| + | |4,10 | ||
| + | |7,8 | ||
| + | |23,0 | ||
| + | | -1,7 | ||
| + | |- | ||
| + | |Molybdän | ||
| + | |0,250 | ||
| + | |900 | ||
| + | |2623 | ||
| + | |292 | ||
| + | |3,6 | ||
| + | |4639 | ||
| + | |5,61 | ||
| + | |138 | ||
| + | |5,2 | ||
| + | | | ||
| + | |- | ||
| + | |Nickel | ||
| + | |0,440 | ||
| + | |520 | ||
| + | |1453 | ||
| + | |301 | ||
| + | |237 | ||
| + | |2913 | ||
| + | |6,45 | ||
| + | |90,7 | ||
| + | |13,0 | ||
| + | | -2,5 | ||
| + | |- | ||
| + | |Niob | ||
| + | |0,272 | ||
| + | | | ||
| + | |2477 | ||
| + | |289 | ||
| + | |7,9x10<sup>-2</sup> | ||
| + | |4744 | ||
| + | |7,79 | ||
| + | |53,7 | ||
| + | |7,3 | ||
| + | | | ||
| + | |- | ||
| + | |Osmium | ||
| + | |0,130 | ||
| + | | | ||
| + | |3045 | ||
| + | |141 | ||
| + | |2,52 | ||
| + | |5012 | ||
| + | |3,81 | ||
| + | |87,6 | ||
| + | |6,5 | ||
| + | | | ||
| + | |- | ||
| + | |Palladium | ||
| + | |0,244 | ||
| + | | | ||
| + | |1554 | ||
| + | |143 | ||
| + | |1,33 | ||
| + | |2970 | ||
| + | |3,48 | ||
| + | |71,8 | ||
| + | |11,1 | ||
| + | | -5,5 | ||
| + | |- | ||
| + | |Platin | ||
| + | |0,130 | ||
| + | |540 | ||
| + | |1772 | ||
| + | |113 | ||
| + | |3,2x10<sup>-2</sup> | ||
| + | |3827 | ||
| + | |2,62 | ||
| + | |71,6 | ||
| + | |9,0 | ||
| + | | -6,0 | ||
| + | |- | ||
| + | |Quecksilber | ||
| + | |0,140 | ||
| + | | | ||
| + | | -38,9 | ||
| + | |11,7 | ||
| + | |3,1x10<sup>-4</sup> | ||
| + | |357 | ||
| + | |0,29 | ||
| + | |8,34 | ||
| + | |60,8 | ||
| + | | -3,7 | ||
| + | |- | ||
| + | |Rhenium | ||
| + | |0,137 | ||
| + | | | ||
| + | |3186 | ||
| + | |178 | ||
| + | |3,24 | ||
| + | |5596 | ||
| + | |3,42 | ||
| + | |72 | ||
| + | |6,7 | ||
| + | | | ||
| + | |- | ||
| + | |Rhodium | ||
| + | |0,242 | ||
| + | | | ||
| + | |1966 | ||
| + | |211 | ||
| + | |6,36x10<sup>-1</sup> | ||
| + | |3695 | ||
| + | |5,19 | ||
| + | |150 | ||
| + | |8,5 | ||
| + | | -10,8 | ||
| + | |- | ||
| + | |Ruthenium | ||
| + | |0,238 | ||
| + | | | ||
| + | |2310 | ||
| + | |252 | ||
| + | |1,4 | ||
| + | |4150 | ||
| + | |6,62 | ||
| + | |117 | ||
| + | |9,5 | ||
| + | | | ||
| + | |- | ||
| + | |Silber | ||
| + | |0,232 | ||
| + | |180 | ||
| + | |961,9 | ||
| + | |105 | ||
| + | |3,4x10<sup>-1</sup> | ||
| + | |2212 | ||
| + | |2,39 | ||
| + | |429 | ||
| + | |19,5 | ||
| + | | -3,8 | ||
| + | |- | ||
| + | |Tantal | ||
| + | |0,140 | ||
| + | |850 | ||
| + | |3017 | ||
| + | |157 | ||
| + | |7,86x10<sup>-1</sup> | ||
| + | |5448 | ||
| + | |4,32 | ||
| + | |57,5 | ||
| + | |6,5 | ||
| + | | | ||
| + | |- | ||
| + | |Titan | ||
| + | |0,520 | ||
| + | | | ||
| + | |1668 | ||
| + | |403 | ||
| + | |4,9x10<sup>-1</sup> | ||
| + | |2830 | ||
| + | |8,80 | ||
| + | |21,9 | ||
| + | |10,8 | ||
| + | | | ||
| + | |- | ||
| + | |Vanadium | ||
| + | |0,490 | ||
| + | | | ||
| + | |1902 | ||
| + | |330 | ||
| + | |3,06 | ||
| + | |3287 | ||
| + | |10,3 | ||
| + | |30,7 | ||
| + | |8,3 | ||
| + | | | ||
| + | |- | ||
| + | |Bismut | ||
| + | |0,122 | ||
| + | | | ||
| + | |271 | ||
| + | |54 | ||
| + | |6,5x10<sup>-4</sup> | ||
| + | |1564 | ||
| + | |1,43 | ||
| + | |7,87 | ||
| + | |14,0 | ||
| + | | -0,33 | ||
| + | |- | ||
| + | |Wolfram | ||
| + | |0,138 | ||
| + | |1000 | ||
| + | |3422 | ||
| + | |193 | ||
| + | |4,27 | ||
| + | |5555 | ||
| + | |3,98 | ||
| + | |174 | ||
| + | |4,5 | ||
| + | | | ||
| + | |- | ||
| + | |Zink | ||
| + | |0,385 | ||
| + | |170 | ||
| + | |420 | ||
| + | |100 | ||
| + | |3,06 | ||
| + | |907 | ||
| + | |1,76 | ||
| + | |116 | ||
| + | |36,0 | ||
| + | | -4,7 | ||
| + | |- | ||
| + | |Zinn | ||
| + | |0,228 | ||
| + | |100 | ||
| + | |222 | ||
| + | |59 | ||
| + | |6x10<sup>-21</sup> | ||
| + | |2602 | ||
| + | |1,95 | ||
| + | |66,6 | ||
| + | |26,7 | ||
| + | | -2,8 | ||
| + | |- | ||
| + | |Zirconium | ||
| + | |0,281 | ||
| + | | | ||
| + | |1852 | ||
| + | |224 | ||
| + | |1,7x10<sup>-3</sup> | ||
| + | |4409 | ||
| + | |4,6 | ||
| + | |22,7 | ||
| + | |5,9 | ||
| + | | | ||
| + | |- | ||
| + | |} | ||
| + | <div id="text-reference"><sub>1</sub> bei 20°C</div> | ||
| + | </figtable> | ||
| + | |||
| + | <figtable id="tab:Elektrische Eigenschaften der wichtigsten Metalle"> | ||
| + | <caption>'''Elektrische Eigenschaften der wichtigsten Metalle'''</caption> | ||
| + | |||
| + | {| class="twocolortable" style="text-align: left; font-size: 12px" | ||
| + | |- | ||
| + | !Element/Metall | ||
| + | !Spezifischer elektrischer Widerstand [[#text-reference|<sup>1</sup>]]<br/>[Ω*mm<sup>2</sup>/m] | ||
| + | !Elektrische Leitfähigkeit<br/>[MS/m] | ||
| + | !Temperaturkoeffizient des<br/>elektrischen Widerstands<br/>[10<sup>-3</sup>/K] | ||
| + | !Kritische Supraleitertemperatur<br/>[K] | ||
| + | !Dämpfungsspannung (gemessen)<br/>[V] | ||
| + | !Schmelzspannung (gemessen)<br/>[V] | ||
| + | !Schmelzspannung (berechnet)<br/>[V] | ||
| + | !Mindestlichtbogenspannung<br/>[V] | ||
| + | !Mindestlichtbogenstrom<br />[A] | ||
| + | |- | ||
| + | |Aluminium | ||
| + | |2,65 | ||
| + | |37,7 | ||
| + | |4,6 | ||
| + | |1,18 | ||
| + | |0,1 | ||
| + | |0,3 | ||
| + | |0,29 | ||
| + | |11,2 | ||
| + | |0,4 | ||
|- | |- | ||
|Antimon | |Antimon | ||
Revision as of 13:33, 16 December 2022
In den nachfolgenden Tabellen sind die physikalischen Eigenschaften der gebräuchlichen
reinen Metalle sowie von Kohlenstoff aufgeführt (Tab. 1 - Tab. 4). Die
Werte können je nach Reinheitsgrad u. U. stark schwanken, teilweise sind sie
auch schwierig zu bestimmen und daher mit Unsicherheiten behaftet. Bei der
Zusammenstellung der Tabellen wurde versucht, aus den Angaben in der Literatur
diejenigen Werte auszuwählen, die als die wahrscheinlichsten anzusehen
sind. Einige Eigenschaften sind anisotrop, d.h. ihre Werte variieren je nach Kristallorientierung.
In solchen Fällen wurden - wenn möglich - die Werte für Vielkristalle
angegeben.
| Element/Metall | Dichte 1
[g/cm³] |
Elastizitätsmodul 1[GPa] | Schubmodul
[GPa] |
Querkontraktionszahl |
|---|---|---|---|---|
| Aluminium | 2.70 | 65 | 27 | 0.34 |
| Antimon | 6.62 | 56 | 20.4 | 0.28 |
| Beryllium | 1.85 | 298 | 150 | 0.12 |
| Blei | 11.36 | 14.5 | 6 | 0.44 |
| Cadmium | 8.65 | 57.5 | 29 | 0.30 |
| Chrom | 7.19 | 160 | 0.25 | |
| Eisen | 7.89 | 208 | 83 | 0.28 |
| Gallium | 5.91 | 9.6 | 0.46 | |
| Gold | 19.32 | 79 | 28 | 0.42 |
| Indium | 7.31 | 11 | 0.45 | |
| Iridium | 22.65 | 538 | 214 | 0.26 |
| Kobalt | 8.85 | 216 | 0.31 | |
| Kohlenstoff (Grafit) | 2.1-2.3 | 5 | ||
| Kupfer | 8.95 | 115 | 48 | 0.34 |
| Magnesium | 1.74 | 46 | 18 | 0.28 |
| Mangan | 7.43 | 165 | 77 | 0.24 |
| Molybdän | 10.21 | 347 | 122 | 0.30 |
| Nickel | 8.90 | 216 | 83 | 0.31 |
| Niob | 8.57 | 113 | 39 | 0.38 |
| Osmium | 22.61 | 570 | 220 | 0.25 |
| Palladium | 12.02 | 124 | 51 | 0.39 |
| Platin | 21.45 | 173 | 67 | 0.39 |
| Quecksilber | 13.55 | |||
| Rhenium | 21.04 | 480 | 215 | 0.26 |
| Rhodium | 12.41 | 386 | 153 | 0.26 |
| Ruthenium | 12.45 | 485 | 172 | 0.29 |
| Silber | 10.49 | 82 | 27 | 0.37 |
| Tantal | 16.60 | 188 | 70 | 0.35 |
| Titan | 4.51 | 120 | 43 | 0.34 |
| Vanadium | 6.10 | 136 | 52 | 0.36 |
| Wismut | 9.80 | 33 | 13 | 0.33 |
| Wolfram | 19.32 | 360 | 158 | 0.30 |
| Zink | 7.13 | 96 | 36 | 0.29 |
| Zinn | 7.30 | 47 | 18 | 0.33 |
| Zirkonium | 6.49 | 98 | 36 | 0.33 |
| Element/Metall | Chemisches Symbol |
Ordnungszahl | Atommasse | Kristallstruktur 1 | Gitterparameter 1 a oder b 2 [1010m] |
Gitterparameter 1 a oder b 2 [10-10m] |
Arbeitsleistung [eV] |
Ionisierungspotenzial [eV] |
|---|---|---|---|---|---|---|---|---|
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | |
| Antimon | Sb | 51 | 121,75 | rhl | 4,507 | 4,1 | 8,64 | |
| Beryllium | Be | 4 | 9,01 | hcp | 2,286 | 3,584 | 3,2 - 3,9 | 9,32 |
| Blei | Pb | 87 | 207,19 | fcc | 4,949 | 4,0 - 4,1 | 7,42 | |
| Cadmium | Cd | 48 | 112,40 | hcp | 2,979 | 5,617 | 3,7 - 4,1 | 8,99 |
| Chrom | Cr | 24 | 52,00 | bcc | 2,884 | 4,4 - 4,7 | 6,76 | |
| Eisen | Fe | 26 | 55,85 | bcc | 2,866 | 4,1 - 4,5 | 7,9 | |
| Gallium | Ga | 31 | 69,72 | ort | 4,524 | 7,661 | 3,8 - 4,1 | 6,0 |
| Gold | Au | 79 | 196,97 | fcc | 4,078 | 4,3 - 5,1 | 9,22 | |
| Indium | In | 49 | 114,82 | tet | 4,594 | 4,951 | 4,0 | 5,79 |
| Iridium | Ir | 77 | 192,20 | fcc | 3,839 | 4,6 - 5,3 | 9,1 | |
| Kobalt | Co | 27 | 58,93 | hcp | 2,507 | 4,069 | 4,4 - 4,6 | 7,86 |
| Kohlenstoff (Graphit) | C | 6 | 12,01 | hcp-Schichtgitter3 | 2,456 | 6,696 | 4,8 | 11,27 |
| Kupfer | Cu | 29 | 63,54 | fcc | 3,615 | 4,4 | 7,72 | |
| Magnesium | Mg | 12 | 24,31 | hcp | 3,209 | 5,210 | 3,7 | 7,64 |
| Mangan | Mn | 25 | 54,94 | kubisch-komplex | 8,912 | 3,8 - 4,1 | 6,0 | |
| Molybdän | Mo | 42 | 95,94 | bcc | 3,147 | 4,1 - 4,5 | 7,18 | |
| Nickel | Ni | 28 | 58,71 | fcc | 3,524 | 5,0 - 5,2 | 7,63 | |
| Niob | Nb | 41 | 92,91 | bcc | 3,301 | 4,0 | 6,77 | |
| Osmium | Os | 76 | 190,23 | hcp | 2,734 | 4,320 | 4,5 | 8,7 |
| Palladium | Pd | 46 | 106,40 | fcc | 3,890 | 4,5 - 5,0 | 8,34 | |
| Platin | Pt | 78 | 195,09 | fcc | 3,931 | 4,1 - 5,5 | 9,0 | |
| Quecksilber | Hg | 80 | 200,59 | rhl4 | 3,0614 | 4,5 | 10,44 | |
| Rhenium | Re | 75 | 186,20 | hcp | 2,760 | 4,458 | 4,7 - 5,0 | 7,8 |
| Rhodium | Rh | 45 | 102,91 | fcc | 3,804 | 4,6 - 4,9 | 7,46 | |
| Ruthenium | Ru | 44 | 101,07 | hcp | 2,704 | 4,281 | 4,5 | 7,37 |
| Silber | Ag | 47 | 107,87 | fcc | 4,086 | 4,3 | 7,57 | |
| Tantal | Ta | 73 | 180,95 | bcc | 3,303 | 4,0 - 4,2 | 7,89 | |
| Titan | Ti | 2 | 47,90 | hcp | 2,950 | 4,683 | 4,0 - 4,4 | 6,83 |
| Vanadium | V | 23 | 50,94 | bcc | 3,039 | 3,8 - 4,2 | 6,71 | |
| Bismut | Bi | 83 | 208,98 | rhl | 4,746 | 4,1 - 4,5 | 8,0 | |
| Wolfram | W | 74 | 183,85 | bcc | 3,158 | 4,3 - 5,0 | 7,98 | |
| Zink | Zn | 30 | 65,37 | hcp | 2,665 | 4,947 | 3,1 - 4,3 | 9,39 |
| Zinn | Sn | 50 | 118,69 | tet | 5,831 | 3,181 | 3,6 - 4,1 | 7,33 |
| Zirconium | Zr | 40 | 91,22 | hcp | 3,231 | 5,148 | 3,7 - 4,3 | 6,92 |
fcc = kubisch-flächenzentriert // bcc = kubisch-körperzentriert // hcp = sechseckig dicht kugelförmig //
ort = orthorhombisch // tet = tetragonal // rhl = rhomboedrisch
| Element/Metall | Spezifische Wärme 1 [kJ/(K*kg)] |
ErweichungsTemperatur [°C] |
Schmelzpunkt [°C] |
Fusionswärme [kJ/kg] |
Dampfdruck beim Schmelzpunkt [Pa] |
Siedepunkt [°C] |
Verdampfungstemperatur [kJ/g] |
Wärmeleitfähigkeit [W/(m*K)] |
Längenausdehnungskoeffizient [10-6m/K] |
Volumenänderung beim Erstarren [%] |
|---|---|---|---|---|---|---|---|---|---|---|
| Aluminium | 0,900 | 150 | 660 | 398 | 2,5x10-6 | 2467 | 10,47 | 237 | 23,6 | -6,5 |
| Antimon | 0,210 | 630 | 163 | 2,5x10-9 | 1587 | 1,97 | 24,3 | 10,5 | +9,5 | |
| Beryllium | 1,824 | 1277 | 1090 | 4,3 | 2477 | 200 | 12,3 | |||
| Blei | 0,130 | 200 | 327 | 25 | 4,21x10-7 | 1750 | 24,70 | 35,3 | 29,3 | -3,5 |
| Cadmium | 0,230 | 321 | 54 | 14,8 | 767 | 0,88 | 96,8 | 41,0 | -4,0 | |
| Chrom | 0,450 | 1857 | 314 | 990 | 2672 | 5,86 | 93,7 | 6,2 | ||
| Eisen | 0,444 | 500 | 1537 | 268 | 7,05 | 2750 | 80,2 | 12,2 | -3,0 | |
| Gallium | 0,370 | 29,8 | 80,4 | 9,6x10-36 | 2204 | 3,90 | 40,6 | 18,0 | +3,0 | |
| Gold | 0,128 | 100 | 1064 | 63 | 2,4x10-3 | 3080 | 1,55 | 317 | 14,3 | -5,1 |
| Indium | 0,233 | 157 | 28,5 | 1,5x10-17 | 2072 | 1,97 | 81,6 | -2,5 | ||
| Iridium | 0,130 | 2410 | 144 | 1,5 | 4130 | 3,31 | 147 | 6,5 | ||
| Cobalt | 0,420 | 1495 | 260 | 175 | 2927 | 6,66 | 100 | 13,8 | ||
| Kohlenstoff (Graphit) | 0,720 | 3825 sublimiert | 119 - 165 | 155 | ||||||
| Kupfer | 0,385 | 190 | 1084 | 205 | 5,2x10-2 | 2567 | 4,77 | 401 | 16,5 | -4,2 |
| Magnesium | 1,020 | 650 | 373 | 361 | 1107 | 5,44 | 156 | 26,0 | -4,1 | |
| Mangan | 0,480 | 1244 | 264 | 121 | 1962 | 4,10 | 7,8 | 23,0 | -1,7 | |
| Molybdän | 0,250 | 900 | 2623 | 292 | 3,6 | 4639 | 5,61 | 138 | 5,2 | |
| Nickel | 0,440 | 520 | 1453 | 301 | 237 | 2913 | 6,45 | 90,7 | 13,0 | -2,5 |
| Niob | 0,272 | 2477 | 289 | 7,9x10-2 | 4744 | 7,79 | 53,7 | 7,3 | ||
| Osmium | 0,130 | 3045 | 141 | 2,52 | 5012 | 3,81 | 87,6 | 6,5 | ||
| Palladium | 0,244 | 1554 | 143 | 1,33 | 2970 | 3,48 | 71,8 | 11,1 | -5,5 | |
| Platin | 0,130 | 540 | 1772 | 113 | 3,2x10-2 | 3827 | 2,62 | 71,6 | 9,0 | -6,0 |
| Quecksilber | 0,140 | -38,9 | 11,7 | 3,1x10-4 | 357 | 0,29 | 8,34 | 60,8 | -3,7 | |
| Rhenium | 0,137 | 3186 | 178 | 3,24 | 5596 | 3,42 | 72 | 6,7 | ||
| Rhodium | 0,242 | 1966 | 211 | 6,36x10-1 | 3695 | 5,19 | 150 | 8,5 | -10,8 | |
| Ruthenium | 0,238 | 2310 | 252 | 1,4 | 4150 | 6,62 | 117 | 9,5 | ||
| Silber | 0,232 | 180 | 961,9 | 105 | 3,4x10-1 | 2212 | 2,39 | 429 | 19,5 | -3,8 |
| Tantal | 0,140 | 850 | 3017 | 157 | 7,86x10-1 | 5448 | 4,32 | 57,5 | 6,5 | |
| Titan | 0,520 | 1668 | 403 | 4,9x10-1 | 2830 | 8,80 | 21,9 | 10,8 | ||
| Vanadium | 0,490 | 1902 | 330 | 3,06 | 3287 | 10,3 | 30,7 | 8,3 | ||
| Bismut | 0,122 | 271 | 54 | 6,5x10-4 | 1564 | 1,43 | 7,87 | 14,0 | -0,33 | |
| Wolfram | 0,138 | 1000 | 3422 | 193 | 4,27 | 5555 | 3,98 | 174 | 4,5 | |
| Zink | 0,385 | 170 | 420 | 100 | 3,06 | 907 | 1,76 | 116 | 36,0 | -4,7 |
| Zinn | 0,228 | 100 | 222 | 59 | 6x10-21 | 2602 | 1,95 | 66,6 | 26,7 | -2,8 |
| Zirconium | 0,281 | 1852 | 224 | 1,7x10-3 | 4409 | 4,6 | 22,7 | 5,9 |
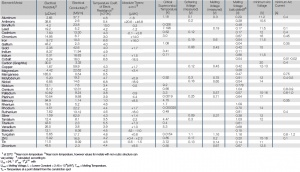
| Element/Metall | Spezifischer elektrischer Widerstand 1 [Ω*mm2/m] |
Elektrische Leitfähigkeit [MS/m] |
Temperaturkoeffizient des elektrischen Widerstands [10-3/K] |
Kritische Supraleitertemperatur [K] |
Dämpfungsspannung (gemessen) [V] |
Schmelzspannung (gemessen) [V] |
Schmelzspannung (berechnet) [V] |
Mindestlichtbogenspannung [V] |
Mindestlichtbogenstrom [A] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Aluminium | 2,65 | 37,7 | 4,6 | 1,18 | 0,1 | 0,3 | 0,29 | 11,2 | 0,4 | |
| Antimon | 0,210 | 630 | 163 | 2,5x10-9 | 1587 | 1,97 | 24,3 | 10,5 | +9,5 | |
| Beryllium | 1,824 | 1277 | 1090 | 4,3 | 2477 | 200 | 12,3 | |||
| Blei | 0,130 | 200 | 327 | 25 | 4,21x10-7 | 1750 | 24,70 | 35,3 | 29,3 | -3,5 |
| Cadmium | 0,230 | 321 | 54 | 14,8 | 767 | 0,88 | 96,8 | 41,0 | -4,0 | |
| Chrom | 0,450 | 1857 | 314 | 990 | 2672 | 5,86 | 93,7 | 6,2 | ||
| Eisen | 0,444 | 500 | 1537 | 268 | 7,05 | 2750 | 80,2 | 12,2 | -3,0 | |
| Gallium | 0,370 | 29,8 | 80,4 | 9,6x10-36 | 2204 | 3,90 | 40,6 | 18,0 | +3,0 | |
| Gold | 0,128 | 100 | 1064 | 63 | 2,4x10-3 | 3080 | 1,55 | 317 | 14,3 | -5,1 |
| Indium | 0,233 | 157 | 28,5 | 1,5x10-17 | 2072 | 1,97 | 81,6 | -2,5 | ||
| Iridium | 0,130 | 2410 | 144 | 1,5 | 4130 | 3,31 | 147 | 6,5 | ||
| Cobalt | 0,420 | 1495 | 260 | 175 | 2927 | 6,66 | 100 | 13,8 | ||
| Kohlenstoff (Graphit) | 0,720 | 3825 sublimiert | 119 - 165 | 155 | ||||||
| Kupfer | 0,385 | 190 | 1084 | 205 | 5,2x10-2 | 2567 | 4,77 | 401 | 16,5 | -4,2 |
| Magnesium | 1,020 | 650 | 373 | 361 | 1107 | 5,44 | 156 | 26,0 | -4,1 | |
| Mangan | 0,480 | 1244 | 264 | 121 | 1962 | 4,10 | 7,8 | 23,0 | -1,7 | |
| Molybdän | 0,250 | 900 | 2623 | 292 | 3,6 | 4639 | 5,61 | 138 | 5,2 | |
| Nickel | 0,440 | 520 | 1453 | 301 | 237 | 2913 | 6,45 | 90,7 | 13,0 | -2,5 |
| Niob | 0,272 | 2477 | 289 | 7,9x10-2 | 4744 | 7,79 | 53,7 | 7,3 | ||
| Osmium | 0,130 | 3045 | 141 | 2,52 | 5012 | 3,81 | 87,6 | 6,5 | ||
| Palladium | 0,244 | 1554 | 143 | 1,33 | 2970 | 3,48 | 71,8 | 11,1 | -5,5 | |
| Platin | 0,130 | 540 | 1772 | 113 | 3,2x10-2 | 3827 | 2,62 | 71,6 | 9,0 | -6,0 |
| Quecksilber | 0,140 | -38,9 | 11,7 | 3,1x10-4 | 357 | 0,29 | 8,34 | 60,8 | -3,7 | |
| Rhenium | 0,137 | 3186 | 178 | 3,24 | 5596 | 3,42 | 72 | 6,7 | ||
| Rhodium | 0,242 | 1966 | 211 | 6,36x10-1 | 3695 | 5,19 | 150 | 8,5 | -10,8 | |
| Ruthenium | 0,238 | 2310 | 252 | 1,4 | 4150 | 6,62 | 117 | 9,5 | ||
| Silber | 0,232 | 180 | 961,9 | 105 | 3,4x10-1 | 2212 | 2,39 | 429 | 19,5 | -3,8 |
| Tantal | 0,140 | 850 | 3017 | 157 | 7,86x10-1 | 5448 | 4,32 | 57,5 | 6,5 | |
| Titan | 0,520 | 1668 | 403 | 4,9x10-1 | 2830 | 8,80 | 21,9 | 10,8 | ||
| Vanadium | 0,490 | 1902 | 330 | 3,06 | 3287 | 10,3 | 30,7 | 8,3 | ||
| Bismut | 0,122 | 271 | 54 | 6,5x10-4 | 1564 | 1,43 | 7,87 | 14,0 | -0,33 | |
| Wolfram | 0,138 | 1000 | 3422 | 193 | 4,27 | 5555 | 3,98 | 174 | 4,5 | |
| Zink | 0,385 | 170 | 420 | 100 | 3,06 | 907 | 1,76 | 116 | 36,0 | -4,7 |
| Zinn | 0,228 | 100 | 222 | 59 | 6x10-21 | 2602 | 1,95 | 66,6 | 26,7 | -2,8 |
| Zirconium | 0,281 | 1852 | 224 | 1,7x10-3 | 4409 | 4,6 | 22,7 | 5,9 |
Referenzen
Metals Handbook, Desk Edition: Chicago, IL, American Society of Metal, 1985
Landolt-Börnstein: Zahlenwerte und Funktionen. Springer-Verlag, Berlin-Göttingen-Heidelberg, 1959
Handbook of Chemistry and Physics, 70th Edition: CRC Press., Inc. Boca Raton, Florida, 1989 - 1990
Fluck, E.; Heumann, K., G.: Periodensystem der Elemente. Weinheim: VCH-Verlagsgesellschaft, 1986
Kieffer, R.; Jangg, G.; Ettmayer, P.: Sondermetalle. Springer- Verlag, Wien-New York, 1963
Hering, E.; Schulz, W.: Physik für Ingenieure (Periodensystem der Elemente). Düsseldorf: VDI-Verlag, 1988
Degussa AG (Hrsg.): Edelmetall-Taschenbuch. Hüthig-Verlag, Heidelberg, 1995
Slade, P.; G. (editor): Electrical Contacts Principles and Applications. Marcel Dekker, Inc., New York-Basel, 1999
Gerritsen, A.; N.: Metallic Conductivity in: Flügge, S.: Handbuch der Physik, Bd. 19, Springer-Verlag, Berlin-Göttingen-Heidelberg, 1956
Köster, W.; Franz, H.: Poisson,s Ratio for Metals and Alloys. Metallurg. Reviews 6 (1961)
Nesmeyanow, A., N.: Vapor Pressure of the Chemical Elements: Elsevier, Amsterdam-London-New York, 1963
Wyckoff, R., W., G.: Crystal Structures. Vol 1,New York, 1963