Difference between revisions of "Physikalische Eigenschaften der wichtigsten Metalle"
Doduco Admin (talk | contribs) |
Doduco Admin (talk | contribs) |
||
| Line 610: | Line 610: | ||
fcc = kubisch-flächenzentriert // bcc = kubisch-körperzentriert // hcp = sechseckig dicht kugelförmig | fcc = kubisch-flächenzentriert // bcc = kubisch-körperzentriert // hcp = sechseckig dicht kugelförmig | ||
ort = orthorhombisch // tet = tetragonal // rhl = rhomboedrisch | ort = orthorhombisch // tet = tetragonal // rhl = rhomboedrisch | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <figtable id="tab:Thermische Eigenschaften der wichtigsten Metalle"> | ||
| + | <caption>'''Thermische Eigenschaften der wichtigsten Metalle'''</caption> | ||
| + | |||
| + | {| class="twocolortable" style="text-align: left; font-size: 12px" | ||
| + | |- | ||
| + | !Element/Metall | ||
| + | !Spezifische Wärme [[#text-reference|<sup>1</sup>]]<br/>[kJ/(K*kg)] | ||
| + | !ErweichungsTemperatur<br/>[°C] | ||
| + | !Schmelzpunkt<br/>[°C] | ||
| + | !Fusionswärme<br/>[kJ/kg] | ||
| + | !Dampfdruck beim Schmelzpunkt<br/>[Pa] | ||
| + | !Siedepunkt<br/>[°C] | ||
| + | !Verdampfungstemperatur<br />[kJ/g] | ||
| + | !Wärmeleitfähigkeit<br/>[W/(m*K)] | ||
| + | !Längenausdehnungskoeffizient<br />[10<sup>-6</sup>m/K] | ||
| + | !Volumenänderung beim Erstarren<br/>[%] | ||
| + | |- | ||
| + | |Aluminium | ||
| + | |0,900 | ||
| + | |150 | ||
| + | |660 | ||
| + | |398 | ||
| + | |2,5x10<sup>-6</sup> | ||
| + | |2467 | ||
| + | |10,47 | ||
| + | |237 | ||
| + | |23,6 | ||
| + | | -6,5 | ||
| + | |- | ||
| + | |Aluminium | ||
| + | |Al | ||
| + | |13 | ||
| + | |26,98 | ||
| + | |foc | ||
| + | |4,049 | ||
| + | | | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |- | ||
| + | |Aluminium | ||
| + | |Al | ||
| + | |13 | ||
| + | |26,98 | ||
| + | |foc | ||
| + | |4,049 | ||
| + | | | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |- | ||
| + | |Aluminium | ||
| + | |Al | ||
| + | |13 | ||
| + | |26,98 | ||
| + | |foc | ||
| + | |4,049 | ||
| + | | | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |- | ||
| + | |Aluminium | ||
| + | |Al | ||
| + | |13 | ||
| + | |26,98 | ||
| + | |foc | ||
| + | |4,049 | ||
| + | | | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |- | ||
| + | |Aluminium | ||
| + | |Al | ||
| + | |13 | ||
| + | |26,98 | ||
| + | |foc | ||
| + | |4,049 | ||
| + | | | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |- | ||
| + | |- | ||
| + | |Aluminium | ||
| + | |Al | ||
| + | |13 | ||
| + | |26,98 | ||
| + | |foc | ||
| + | |4,049 | ||
| + | | | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |- | ||
| + | |Aluminium | ||
| + | |Al | ||
| + | |13 | ||
| + | |26,98 | ||
| + | |foc | ||
| + | |4,049 | ||
| + | | | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |- | ||
| + | |Aluminium | ||
| + | |Al | ||
| + | |13 | ||
| + | |26,98 | ||
| + | |foc | ||
| + | |4,049 | ||
| + | | | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |- | ||
| + | |Indium | ||
| + | |In | ||
| + | |49 | ||
| + | |114,82 | ||
| + | |tet | ||
| + | |4,594 | ||
| + | |4,951 | ||
| + | |4,0 | ||
| + | |5,79 | ||
| + | |- | ||
| + | |Iridium | ||
| + | |Ir | ||
| + | |77 | ||
| + | |192,20 | ||
| + | |fcc | ||
| + | |3,839 | ||
| + | | | ||
| + | |4,6 - 5,3 | ||
| + | |9,1 | ||
| + | |- | ||
| + | |Kobalt | ||
| + | |Co | ||
| + | |27 | ||
| + | |58,93 | ||
| + | |hcp | ||
| + | |2,507 | ||
| + | |4,069 | ||
| + | |4,4 - 4,6 | ||
| + | |7,86 | ||
| + | |- | ||
| + | |Kohlenstoff (Graphit) | ||
| + | |C | ||
| + | |6 | ||
| + | |12,01 | ||
| + | |hcp-Schichtgitter[[#text-reference|<sup>3</sup>]] | ||
| + | |2,456 | ||
| + | |6,696 | ||
| + | |4,8 | ||
| + | |11,27 | ||
| + | |- | ||
| + | |Kupfer | ||
| + | |Cu | ||
| + | |29 | ||
| + | |63,54 | ||
| + | |fcc | ||
| + | |3,615 | ||
| + | | | ||
| + | |4,4 | ||
| + | |7,72 | ||
| + | |- | ||
| + | |Magnesium | ||
| + | |Mg | ||
| + | |12 | ||
| + | |24,31 | ||
| + | |hcp | ||
| + | |3,209 | ||
| + | |5,210 | ||
| + | |3,7 | ||
| + | |7,64 | ||
| + | |- | ||
| + | |Mangan | ||
| + | |Mn | ||
| + | |25 | ||
| + | |54,94 | ||
| + | |kubisch-komplex | ||
| + | |8,912 | ||
| + | | | ||
| + | |3,8 - 4,1 | ||
| + | |6,0 | ||
| + | |- | ||
| + | |Molybdän | ||
| + | |Mo | ||
| + | |42 | ||
| + | |95,94 | ||
| + | |bcc | ||
| + | |3,147 | ||
| + | | | ||
| + | |4,1 - 4,5 | ||
| + | |7,18 | ||
| + | |- | ||
| + | |Nickel | ||
| + | |Ni | ||
| + | |28 | ||
| + | |58,71 | ||
| + | |fcc | ||
| + | |3,524 | ||
| + | | | ||
| + | |5,0 - 5,2 | ||
| + | |7,63 | ||
| + | |- | ||
| + | |Niob | ||
| + | |Nb | ||
| + | |41 | ||
| + | |92,91 | ||
| + | |bcc | ||
| + | |3,301 | ||
| + | | | ||
| + | |4,0 | ||
| + | |6,77 | ||
| + | |- | ||
| + | |Osmium | ||
| + | |Os | ||
| + | |76 | ||
| + | |190,23 | ||
| + | |hcp | ||
| + | |2,734 | ||
| + | |4,320 | ||
| + | |4,5 | ||
| + | |8,7 | ||
| + | |- | ||
| + | |Palladium | ||
| + | |Pd | ||
| + | |46 | ||
| + | |106,40 | ||
| + | |fcc | ||
| + | |3,890 | ||
| + | | | ||
| + | |4,5 - 5,0 | ||
| + | |8,34 | ||
| + | |- | ||
| + | |Platin | ||
| + | |Pt | ||
| + | |78 | ||
| + | |195,09 | ||
| + | |fcc | ||
| + | |3,931 | ||
| + | | | ||
| + | |4,1 - 5,5 | ||
| + | |9,0 | ||
| + | |- | ||
| + | |Quecksilber | ||
| + | |Hg | ||
| + | |80 | ||
| + | |200,59 | ||
| + | |rhl[[#text-reference|<sup>4</sup>]] | ||
| + | |3,061[[#text-reference|<sup>4</sup>]] | ||
| + | | | ||
| + | |4,5 | ||
| + | |10,44 | ||
| + | |- | ||
| + | |Rhenium | ||
| + | |Re | ||
| + | |75 | ||
| + | |186,20 | ||
| + | |hcp | ||
| + | |2,760 | ||
| + | |4,458 | ||
| + | |4,7 - 5,0 | ||
| + | |7,8 | ||
| + | |- | ||
| + | |Rhodium | ||
| + | |Rh | ||
| + | |45 | ||
| + | |102,91 | ||
| + | |fcc | ||
| + | |3,804 | ||
| + | | | ||
| + | |4,6 - 4,9 | ||
| + | |7,46 | ||
| + | |- | ||
| + | |Ruthenium | ||
| + | |Ru | ||
| + | |44 | ||
| + | |101,07 | ||
| + | |hcp | ||
| + | |2,704 | ||
| + | |4,281 | ||
| + | |4,5 | ||
| + | |7,37 | ||
| + | |- | ||
| + | |Silber | ||
| + | |Ag | ||
| + | |47 | ||
| + | |107,87 | ||
| + | |fcc | ||
| + | |4,086 | ||
| + | | | ||
| + | |4,3 | ||
| + | |7,57 | ||
| + | |- | ||
| + | |Tantal | ||
| + | |Ta | ||
| + | |73 | ||
| + | |180,95 | ||
| + | |bcc | ||
| + | |3,303 | ||
| + | | | ||
| + | |4,0 - 4,2 | ||
| + | |7,89 | ||
| + | |- | ||
| + | |Titan | ||
| + | |Ti | ||
| + | |2 | ||
| + | |47,90 | ||
| + | |hcp | ||
| + | |2,950 | ||
| + | |4,683 | ||
| + | |4,0 - 4,4 | ||
| + | |6,83 | ||
| + | |- | ||
| + | |Vanadium | ||
| + | |V | ||
| + | |23 | ||
| + | |50,94 | ||
| + | |bcc | ||
| + | |3,039 | ||
| + | | | ||
| + | |3,8 - 4,2 | ||
| + | |6,71 | ||
| + | |- | ||
| + | |Bismut | ||
| + | |Bi | ||
| + | |83 | ||
| + | |208,98 | ||
| + | |rhl | ||
| + | |4,746 | ||
| + | | | ||
| + | |4,1 - 4,5 | ||
| + | |8,0 | ||
| + | |- | ||
| + | |Wolfram | ||
| + | |W | ||
| + | |74 | ||
| + | |183,85 | ||
| + | |bcc | ||
| + | |3,158 | ||
| + | | | ||
| + | |4,3 - 5,0 | ||
| + | |7,98 | ||
| + | |- | ||
| + | |Zink | ||
| + | |Zn | ||
| + | |30 | ||
| + | |65,37 | ||
| + | |hcp | ||
| + | |2,665 | ||
| + | |4,947 | ||
| + | |3,1 - 4,3 | ||
| + | |9,39 | ||
| + | |- | ||
| + | |Zinn | ||
| + | |Sn | ||
| + | |50 | ||
| + | |118,69 | ||
| + | |tet | ||
| + | |5,831 | ||
| + | |3,181 | ||
| + | |3,6 - 4,1 | ||
| + | |7,33 | ||
| + | |- | ||
| + | |Zirconium | ||
| + | |Zr | ||
| + | |40 | ||
| + | |91,22 | ||
| + | |hcp | ||
| + | |3,231 | ||
| + | |5,148 | ||
| + | |3,7 - 4,3 | ||
| + | |6,92 | ||
| + | |- | ||
| + | |} | ||
| + | <div id="text-reference"><sub>1</sub> bei 20°C</div> | ||
Revision as of 10:02, 16 December 2022
In den nachfolgenden Tabellen sind die physikalischen Eigenschaften der gebräuchlichen
reinen Metalle sowie von Kohlenstoff aufgeführt (Tab. 1 - Tab. 4). Die
Werte können je nach Reinheitsgrad u. U. stark schwanken, teilweise sind sie
auch schwierig zu bestimmen und daher mit Unsicherheiten behaftet. Bei der
Zusammenstellung der Tabellen wurde versucht, aus den Angaben in der Literatur
diejenigen Werte auszuwählen, die als die wahrscheinlichsten anzusehen
sind. Einige Eigenschaften sind anisotrop, d.h. ihre Werte variieren je nach Kristallorientierung.
In solchen Fällen wurden - wenn möglich - die Werte für Vielkristalle
angegeben.
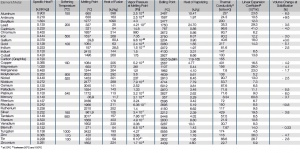
| Element/Metall | Dichte 1
[g/cm³] |
Elastizitätsmodul 1[GPa] | Schubmodul
[GPa] |
Querkontraktionszahl |
|---|---|---|---|---|
| Aluminium | 2.70 | 65 | 27 | 0.34 |
| Antimon | 6.62 | 56 | 20.4 | 0.28 |
| Beryllium | 1.85 | 298 | 150 | 0.12 |
| Blei | 11.36 | 14.5 | 6 | 0.44 |
| Cadmium | 8.65 | 57.5 | 29 | 0.30 |
| Chrom | 7.19 | 160 | 0.25 | |
| Eisen | 7.89 | 208 | 83 | 0.28 |
| Gallium | 5.91 | 9.6 | 0.46 | |
| Gold | 19.32 | 79 | 28 | 0.42 |
| Indium | 7.31 | 11 | 0.45 | |
| Iridium | 22.65 | 538 | 214 | 0.26 |
| Kobalt | 8.85 | 216 | 0.31 | |
| Kohlenstoff (Grafit) | 2.1-2.3 | 5 | ||
| Kupfer | 8.95 | 115 | 48 | 0.34 |
| Magnesium | 1.74 | 46 | 18 | 0.28 |
| Mangan | 7.43 | 165 | 77 | 0.24 |
| Molybdän | 10.21 | 347 | 122 | 0.30 |
| Nickel | 8.90 | 216 | 83 | 0.31 |
| Niob | 8.57 | 113 | 39 | 0.38 |
| Osmium | 22.61 | 570 | 220 | 0.25 |
| Palladium | 12.02 | 124 | 51 | 0.39 |
| Platin | 21.45 | 173 | 67 | 0.39 |
| Quecksilber | 13.55 | |||
| Rhenium | 21.04 | 480 | 215 | 0.26 |
| Rhodium | 12.41 | 386 | 153 | 0.26 |
| Ruthenium | 12.45 | 485 | 172 | 0.29 |
| Silber | 10.49 | 82 | 27 | 0.37 |
| Tantal | 16.60 | 188 | 70 | 0.35 |
| Titan | 4.51 | 120 | 43 | 0.34 |
| Vanadium | 6.10 | 136 | 52 | 0.36 |
| Wismut | 9.80 | 33 | 13 | 0.33 |
| Wolfram | 19.32 | 360 | 158 | 0.30 |
| Zink | 7.13 | 96 | 36 | 0.29 |
| Zinn | 7.30 | 47 | 18 | 0.33 |
| Zirkonium | 6.49 | 98 | 36 | 0.33 |
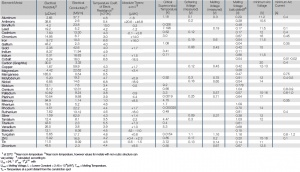
| Element/Metall | Chemisches Symbol |
Ordnungszahl | Atommasse | Kristallstruktur 1 | Gitterparameter 1 a oder b 2 [1010m] |
Gitterparameter 1 a oder b 2 [10-10m] |
Arbeitsleistung [eV] |
Ionisierungspotenzial [eV] |
|---|---|---|---|---|---|---|---|---|
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | |
| Antimon | Sb | 51 | 121,75 | rhl | 4,507 | 4,1 | 8,64 | |
| Beryllium | Be | 4 | 9,01 | hcp | 2,286 | 3,584 | 3,2 - 3,9 | 9,32 |
| Blei | Pb | 87 | 207,19 | fcc | 4,949 | 4,0 - 4,1 | 7,42 | |
| Cadmium | Cd | 48 | 112,40 | hcp | 2,979 | 5,617 | 3,7 - 4,1 | 8,99 |
| Chrom | Cr | 24 | 52,00 | bcc | 2,884 | 4,4 - 4,7 | 6,76 | |
| Eisen | Fe | 26 | 55,85 | bcc | 2,866 | 4,1 - 4,5 | 7,9 | |
| Gallium | Ga | 31 | 69,72 | ort | 4,524 | 7,661 | 3,8 - 4,1 | 6,0 |
| Gold | Au | 79 | 196,97 | fcc | 4,078 | 4,3 - 5,1 | 9,22 | |
| Indium | In | 49 | 114,82 | tet | 4,594 | 4,951 | 4,0 | 5,79 |
| Iridium | Ir | 77 | 192,20 | fcc | 3,839 | 4,6 - 5,3 | 9,1 | |
| Kobalt | Co | 27 | 58,93 | hcp | 2,507 | 4,069 | 4,4 - 4,6 | 7,86 |
| Kohlenstoff (Graphit) | C | 6 | 12,01 | hcp-Schichtgitter3 | 2,456 | 6,696 | 4,8 | 11,27 |
| Kupfer | Cu | 29 | 63,54 | fcc | 3,615 | 4,4 | 7,72 | |
| Magnesium | Mg | 12 | 24,31 | hcp | 3,209 | 5,210 | 3,7 | 7,64 |
| Mangan | Mn | 25 | 54,94 | kubisch-komplex | 8,912 | 3,8 - 4,1 | 6,0 | |
| Molybdän | Mo | 42 | 95,94 | bcc | 3,147 | 4,1 - 4,5 | 7,18 | |
| Nickel | Ni | 28 | 58,71 | fcc | 3,524 | 5,0 - 5,2 | 7,63 | |
| Niob | Nb | 41 | 92,91 | bcc | 3,301 | 4,0 | 6,77 | |
| Osmium | Os | 76 | 190,23 | hcp | 2,734 | 4,320 | 4,5 | 8,7 |
| Palladium | Pd | 46 | 106,40 | fcc | 3,890 | 4,5 - 5,0 | 8,34 | |
| Platin | Pt | 78 | 195,09 | fcc | 3,931 | 4,1 - 5,5 | 9,0 | |
| Quecksilber | Hg | 80 | 200,59 | rhl4 | 3,0614 | 4,5 | 10,44 | |
| Rhenium | Re | 75 | 186,20 | hcp | 2,760 | 4,458 | 4,7 - 5,0 | 7,8 |
| Rhodium | Rh | 45 | 102,91 | fcc | 3,804 | 4,6 - 4,9 | 7,46 | |
| Ruthenium | Ru | 44 | 101,07 | hcp | 2,704 | 4,281 | 4,5 | 7,37 |
| Silber | Ag | 47 | 107,87 | fcc | 4,086 | 4,3 | 7,57 | |
| Tantal | Ta | 73 | 180,95 | bcc | 3,303 | 4,0 - 4,2 | 7,89 | |
| Titan | Ti | 2 | 47,90 | hcp | 2,950 | 4,683 | 4,0 - 4,4 | 6,83 |
| Vanadium | V | 23 | 50,94 | bcc | 3,039 | 3,8 - 4,2 | 6,71 | |
| Bismut | Bi | 83 | 208,98 | rhl | 4,746 | 4,1 - 4,5 | 8,0 | |
| Wolfram | W | 74 | 183,85 | bcc | 3,158 | 4,3 - 5,0 | 7,98 | |
| Zink | Zn | 30 | 65,37 | hcp | 2,665 | 4,947 | 3,1 - 4,3 | 9,39 |
| Zinn | Sn | 50 | 118,69 | tet | 5,831 | 3,181 | 3,6 - 4,1 | 7,33 |
| Zirconium | Zr | 40 | 91,22 | hcp | 3,231 | 5,148 | 3,7 - 4,3 | 6,92 |
fcc = kubisch-flächenzentriert // bcc = kubisch-körperzentriert // hcp = sechseckig dicht kugelförmig
ort = orthorhombisch // tet = tetragonal // rhl = rhomboedrisch
| Element/Metall | Spezifische Wärme 1 [kJ/(K*kg)] |
ErweichungsTemperatur [°C] |
Schmelzpunkt [°C] |
Fusionswärme [kJ/kg] |
Dampfdruck beim Schmelzpunkt [Pa] |
Siedepunkt [°C] |
Verdampfungstemperatur [kJ/g] |
Wärmeleitfähigkeit [W/(m*K)] |
Längenausdehnungskoeffizient [10-6m/K] |
Volumenänderung beim Erstarren [%] |
|---|---|---|---|---|---|---|---|---|---|---|
| Aluminium | 0,900 | 150 | 660 | 398 | 2,5x10-6 | 2467 | 10,47 | 237 | 23,6 | -6,5 |
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | 4,08 - 4,3 | 5,98 | |
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | 4,08 - 4,3 | 5,98 | |
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | 4,08 - 4,3 | 5,98 | |
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | 4,08 - 4,3 | 5,98 | |
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | 4,08 - 4,3 | 5,98 | |
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | 4,08 - 4,3 | 5,98 | |
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | 4,08 - 4,3 | 5,98 | |
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | 4,08 - 4,3 | 5,98 | |
| Indium | In | 49 | 114,82 | tet | 4,594 | 4,951 | 4,0 | 5,79 | ||
| Iridium | Ir | 77 | 192,20 | fcc | 3,839 | 4,6 - 5,3 | 9,1 | |||
| Kobalt | Co | 27 | 58,93 | hcp | 2,507 | 4,069 | 4,4 - 4,6 | 7,86 | ||
| Kohlenstoff (Graphit) | C | 6 | 12,01 | hcp-Schichtgitter3 | 2,456 | 6,696 | 4,8 | 11,27 | ||
| Kupfer | Cu | 29 | 63,54 | fcc | 3,615 | 4,4 | 7,72 | |||
| Magnesium | Mg | 12 | 24,31 | hcp | 3,209 | 5,210 | 3,7 | 7,64 | ||
| Mangan | Mn | 25 | 54,94 | kubisch-komplex | 8,912 | 3,8 - 4,1 | 6,0 | |||
| Molybdän | Mo | 42 | 95,94 | bcc | 3,147 | 4,1 - 4,5 | 7,18 | |||
| Nickel | Ni | 28 | 58,71 | fcc | 3,524 | 5,0 - 5,2 | 7,63 | |||
| Niob | Nb | 41 | 92,91 | bcc | 3,301 | 4,0 | 6,77 | |||
| Osmium | Os | 76 | 190,23 | hcp | 2,734 | 4,320 | 4,5 | 8,7 | ||
| Palladium | Pd | 46 | 106,40 | fcc | 3,890 | 4,5 - 5,0 | 8,34 | |||
| Platin | Pt | 78 | 195,09 | fcc | 3,931 | 4,1 - 5,5 | 9,0 | |||
| Quecksilber | Hg | 80 | 200,59 | rhl4 | 3,0614 | 4,5 | 10,44 | |||
| Rhenium | Re | 75 | 186,20 | hcp | 2,760 | 4,458 | 4,7 - 5,0 | 7,8 | ||
| Rhodium | Rh | 45 | 102,91 | fcc | 3,804 | 4,6 - 4,9 | 7,46 | |||
| Ruthenium | Ru | 44 | 101,07 | hcp | 2,704 | 4,281 | 4,5 | 7,37 | ||
| Silber | Ag | 47 | 107,87 | fcc | 4,086 | 4,3 | 7,57 | |||
| Tantal | Ta | 73 | 180,95 | bcc | 3,303 | 4,0 - 4,2 | 7,89 | |||
| Titan | Ti | 2 | 47,90 | hcp | 2,950 | 4,683 | 4,0 - 4,4 | 6,83 | ||
| Vanadium | V | 23 | 50,94 | bcc | 3,039 | 3,8 - 4,2 | 6,71 | |||
| Bismut | Bi | 83 | 208,98 | rhl | 4,746 | 4,1 - 4,5 | 8,0 | |||
| Wolfram | W | 74 | 183,85 | bcc | 3,158 | 4,3 - 5,0 | 7,98 | |||
| Zink | Zn | 30 | 65,37 | hcp | 2,665 | 4,947 | 3,1 - 4,3 | 9,39 | ||
| Zinn | Sn | 50 | 118,69 | tet | 5,831 | 3,181 | 3,6 - 4,1 | 7,33 | ||
| Zirconium | Zr | 40 | 91,22 | hcp | 3,231 | 5,148 | 3,7 - 4,3 | 6,92 |
<figtable id="tab:Thermal Properties of the Most Important Metals">
Referenzen
Metals Handbook, Desk Edition: Chicago, IL, American Society of Metal, 1985
Landolt-Börnstein: Zahlenwerte und Funktionen. Springer-Verlag, Berlin-Göttingen-Heidelberg, 1959
Handbook of Chemistry and Physics, 70th Edition: CRC Press., Inc. Boca Raton, Florida, 1989 - 1990
Fluck, E.; Heumann, K., G.: Periodensystem der Elemente. Weinheim: VCH-Verlagsgesellschaft, 1986
Kieffer, R.; Jangg, G.; Ettmayer, P.: Sondermetalle. Springer- Verlag, Wien-New York, 1963
Hering, E.; Schulz, W.: Physik für Ingenieure (Periodensystem der Elemente). Düsseldorf: VDI-Verlag, 1988
Degussa AG (Hrsg.): Edelmetall-Taschenbuch. Hüthig-Verlag, Heidelberg, 1995
Slade, P.; G. (editor): Electrical Contacts Principles and Applications. Marcel Dekker, Inc., New York-Basel, 1999
Gerritsen, A.; N.: Metallic Conductivity in: Flügge, S.: Handbuch der Physik, Bd. 19, Springer-Verlag, Berlin-Göttingen-Heidelberg, 1956
Köster, W.; Franz, H.: Poisson,s Ratio for Metals and Alloys. Metallurg. Reviews 6 (1961)
Nesmeyanow, A., N.: Vapor Pressure of the Chemical Elements: Elsevier, Amsterdam-London-New York, 1963
Wyckoff, R., W., G.: Crystal Structures. Vol 1,New York, 1963