Difference between revisions of "Platinum Metal Based Materials"
Doduco Admin (talk | contribs) |
Doduco Admin (talk | contribs) |
||
| Line 51: | Line 51: | ||
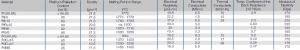
Alloys of Pt with Ru, Ir, Ni, and W were widely used in electromechanical components in the telecommunication industry and in heavy duty automotive breaker points <xr id="fig:Physical Properties of platinum metals"/> (Tab. 2.7). | Alloys of Pt with Ru, Ir, Ni, and W were widely used in electromechanical components in the telecommunication industry and in heavy duty automotive breaker points <xr id="fig:Physical Properties of platinum metals"/> (Tab. 2.7). | ||
| − | <figure id="fig:Physical Properties of platinum metals"> | + | <figure id="fig:Physical Properties of platinum metals" title="<caption>Physical Properties of platinum metals</caption>"> |
[[File:Physical Properties of platinum metals.jpg|right|thumb|Physical Properties of the Platinum Metals and their Alloys]] | [[File:Physical Properties of platinum metals.jpg|right|thumb|Physical Properties of the Platinum Metals and their Alloys]] | ||
</figure> | </figure> | ||
Revision as of 16:28, 30 April 2014
The platinum group metals include the elements Pt, Pd, Rh, Ru, Ir, and Os Table 1 (Tab. 2.6). For electrical contacts platinum and palladium have practical significance as base alloy materials and ruthenium and iridium are used as alloying components. Pt and Pd have similar corrosion resistance as gold but because of their catalytical properties they tend to polymerize adsorbed organic vapors on contact surfaces. During frictional movement between contact surfaces the polymerized compounds known as “brown powder” are formed which can lead to significantly increase in contact resistance. Therefore Pt and Pd are typically used as alloys and not in their pure form for electrical contact applications.
| Element | Properties | Processing | Forms of Application |
|---|---|---|---|
| Ru Ruthenium |
Dull grey to silvery white, very hard and brittle, in the presence of oxygen resistant to acids, oxidizes during heating in air |
Vapor deposition, sputtering, powder metallurgy, warm-forming only possible at 1200 – 1500°C |
Powder; in sheet form, as coatings, and as wire mostly as alloying component |
| Rh Rhodium |
Almost silvery white, very hard and brittle, not soluble in acids, oxidizes in air during red anneal |
Electroplating, vapor deposition, sputtering, after warm-forming at 800 – 1000°C cold working is possible |
Coatings (electroplated), alloying component, in limited form as sheet and wire |
| Pd Palladium |
Dull white, resistant to most acids, oxidizes at red anneal |
Electroplating, vapor deposition, sputtering, cold working |
Sheet, strip, tubing, wire, rivets, and coatings |
| Os Osmium |
Bluish white, hardest platinum metal, very brittle, resistant against non-oxidizing acids, oxidizes easily on air |
Powder metallurgy | Powder, alloying component |
| Ir Iridium |
Almost silvery white, very hard and brittle, acid resistant, oxidizes at red anneal |
Vapor deposition, sputtering, powder metallurgy, warm-forming possible at 1200 – 1500°C |
Powder, alloying component, in limited amounts as sheet |
| Pt Platinum |
Grey white, ductile, acid resistant except for aqua regia, HBr, and HJ, oxidation resistant at red anneal |
Electroplating, vapor deposition, sputtering, cold working |
Sheet, strip, tubing, wire, rivets, coatings |
Rhodium is not used as a solid contact material but is applied for example as a electroplated layer in sliding contact systems. Ruthenium is mostly used as an alloying component in the material PdRu15. The metals osmium and iridium have no practical applications in electrical contacts.
Since Pd was for the longest time rather stable in price it was looked at as a substitute for the more expensive gold. This was followed by a steep increase in the Pd price which caused a significant reduction in its use in electrical contacts. Today (2011) the Pd price again is lower than that of gold.
Alloys of Pt with Ru, Ir, Ni, and W were widely used in electromechanical components in the telecommunication industry and in heavy duty automotive breaker points Figure 1 (Tab. 2.7).
Today these components have been replaced in many applications by solid state technology and the usage of these materials is greatly reduced. Pd alloys however have a more significant importance. PdCu15 is widely used for example in automotive flasher relays. Because of their resistance to sulfide formation PdAg alloys are applied in various relay designs. The ability to thermally precipitation harden some multi component alloys based on PdAgAuPt they find special usage in wear resistant sliding contact applications. Pd44Ag38Cu15PtAuZn is a standard alloy in this group Table 2 (Tab 2.8) und Table 3 (Tab. 2.9)
Platinum and palladium alloys are mainly used similar to the gold based materials in the form of welded wire and profile segments but rarely as contact rivets. Because of the high precious metal prices joining technologies are used that allow the most economic application of the contact alloy in the area where functionally needed. Because of their resistance to material transfer they are used for DC applications and due to their higher arc erosion resistance they are applied for medium electrical loads up to about 30W in relays and switches Table 4 (Table 2.10). Multi-component alloys based on Pd with higher hardness and wear resistance are mainly used as spring arms in sliding contact systems and DC miniature motors.
| Material | Tensile Strength [MPa] | Elongation A [%] | Vickers Hardness HV 1 | |||
|---|---|---|---|---|---|---|
| soft | 70% cold worket | soft | 70% cold worket | soft | 70% cold worket | |
| Pt (99,95) | 150 | 360 | 40 | 3 | 40 | 120 |
| PtIr5 | 260 | 550 | 25 | 2 | 85 | 160 |
| PtIr10 | 340 | 570 | 24 | 2 | 105 | 210 |
| PtRu10 | 650 | 1000 | 24 | 2 | 195 | 320 |
| PtNi8 | 640 | 950 | 22 | 2 | 200 | 320 |
| PtW5 | 530 | 860 | 21 | 2 | 150 | 270 |
| Pd (99,95) | 200 | 420 | 42 | 2 | 40 | 90 |
| PdCu15 | 400 | 780 | 38 | 2 | 90 | 220 |
| PdCu40 | 550 | 950 | 35 | 2 | 120 | 260 |
| PdNi5 | 340 | 700 | 25 | 2 | 95 | 200 |
| Pd35AuAgPt | 420* | |||||
| Pd44Ag38Cu15 PtAuZn | 405* | |||||
| Pd40Co40W20 | 680* | |||||
- maximum hardness
Table 3: Table 2.9: Contact and Switching Properties of the Platinum Metals and their Alloys
Material | Properties | ||
|---|---|---|---|
Pt | Very high corrosion resistance | ||
PtIr5 - 10 | Very high corrosion resistance, low contact resistance | High arc erosion resistance, high hardness | |
PtRu10 | Very high corrosion resistance, low welding tendency | Low contact resistance, very high hardness | |
PtNi8 | Low material transfer tendency | Very high hardness | |
PtW5 | Low material transfer tendency | High hardness | |
Pd | Strong tendency to “Brown Powder” formation | Less arc erosion resistant than Pt | |
PdCu15 PdCu40 | Tendency to “Brown Powder” formation | Mostly resistant to material transfer, high hardness | |
PdNi5 | Strong tendency to “Brown Powder” formation | Low welding tendency | |
Pd44Ag38Cu15 PtAuZn | High mechanical wear resistance | Standard material for sliding contact brushes | |
Table 4: Table 2.10: Application Examples and Form of Supply for Platinum Metals and their Alloys
Material | Application Examples | Forms of Supply |
|---|---|---|
Pt (99,95) | Relays | Contact rivets, welded contact parts |
PtIr5 PtIr10 PtRu10 PtNi8 PtW5 | Relays, sliding contact systems, automotive ignition breaker points | Semi-finished Contact Materials: Wire, seam-welded contact profiles Contact Parts: Tips, wire-formed parts, solid and composite contact rivets, welded contact parts |
Pd (99,95) PdNi5 | Relays | Micro-profiles (weld tapes), contact rivets, welded contact parts |
PdCu15 PdCu40 | Automotive flasher relays | Micro-profiles, composite contact rivets |
Pd35AuAgPt Pd44Ag38Cu15 PtAuZn Pd40Co40W20 | Potentiometers, slip rings, miniature DC motors | Wire-formed parts, welded wire segments, multi-arm sliding contact brushes |
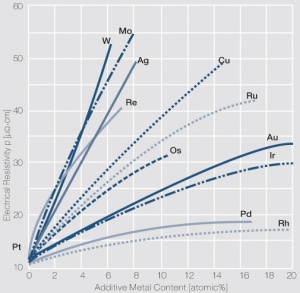
Figure 2Influence of 1-20 atom% of different additive metals on the electrical resistivity p of platinum (Degussa)
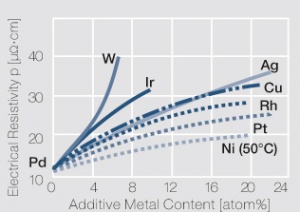
Figure 3 Influence of 1-22 atom% of different additive metals on the electrical resistivity p of palladium
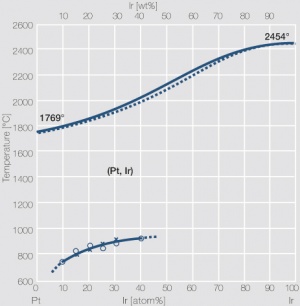
Figure 4Fig. 2.27: Phase diagram of platinum-iridium
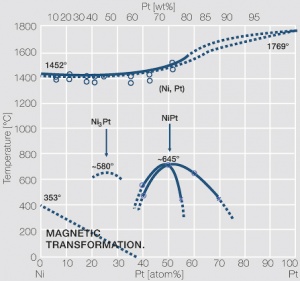
Figure 5 Fig. 2.28: Phase diagram of platinum-nickel
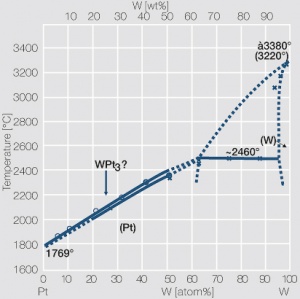
Figure 6 Fig. 2.29: Phase diagram of platinum-tungsten
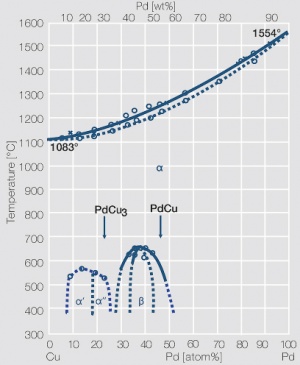
Figure 7 Fig. 2.30: Phase diagram of palladium-copper
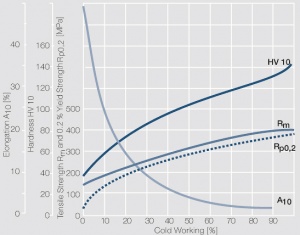
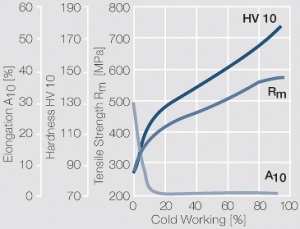
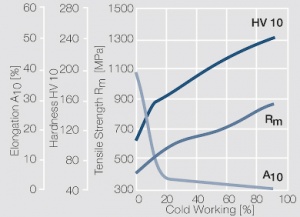
Figure 8 Fig. 2.31: Strain hardening of Pt by cold working
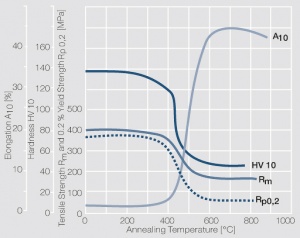
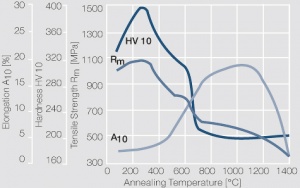
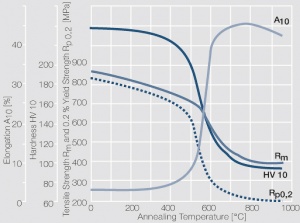
Figure 9 Fig. 2.32: Softening of Pt after annealing for 0.5 hrs after 80% cold working
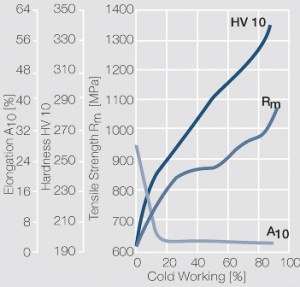
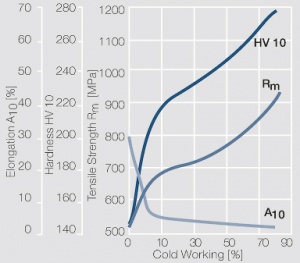
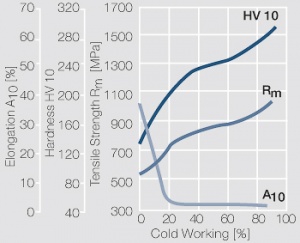
Figure 10 Fig. 2.33: Strain hardening of PtIr5 by cold working
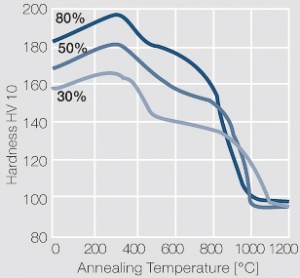
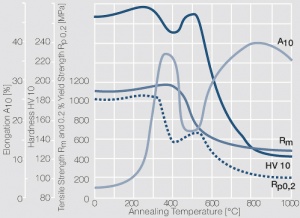
Figure 11 Fig. 2.34: Softening of PtIr5 after annealing for 1 hr after different degrees of cold working
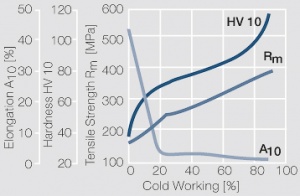
Figure 12Fig. 2.35: Strain hardening of PtNi8 by cold working
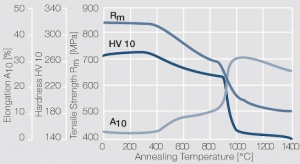
Figure 13 Fig. 2.36: Softening of PtNi8 after annealing for 1 hr after 80% cold working
Figure 14Fig. 2.37: Strain hardening of PtW5 by cold working
Figure 15Fig. 2.38: Softening of PtW5 after annealing for 1hr after 80% cold working
Figure 16Fig. 2.39: Strain hardening of Pd 99.99 by cold working
Figure 17Fig. 2.40: Strain hardening of PdCu15 by cold working
Figure 18Fig. 2.41: Softening of PdCu15 after annealing for 0.5 hrs
Figure 19Fig. 2.42: Strain hardening of PdCu40 by cold working
Figure 20Fig. 2.43: Softening of PdCu40 after annealing for 0.5 hrs after 80% cold working
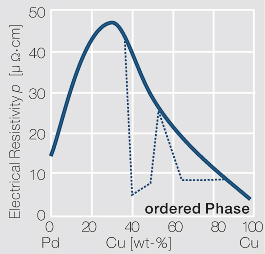
Figure 21Fig. 2.44: Electrical resistivity p of PdCu alloys with and without an annealing step for forming an ordered phase