Difference between revisions of "Brazing Alloys and Fluxes"
(→Brazing Alloys) |
(→Fluxes) |
||
| Line 16: | Line 16: | ||
== Fluxes == | == Fluxes == | ||
| − | Brazing fluxes consist of non-metallic materials, mostly salt mixtures of boron and halogen compounds <xr id="tab: | + | Brazing fluxes consist of non-metallic materials, mostly salt mixtures of boron and halogen compounds <xr id="tab:Fluxes for the Brazing of Heavy Metals"/> (Table 4.2). Their purpose is to remove oxides from the brazing surfaces and prevent their new build-up in order to allow a thorough wetting of these surfaces by the liquefied brazing alloy. Fluxes have to be activated already at a temperature below the working range of the brazing alloy. They are selected mainly according to the working temperature of the brazing alloy and the base material to be joined. |
Since the residues of fluxes are hygroscopic and can cause corrosion they have to be removed completely after the brazing process in very hot or boiling water. Depending on the type and process used, fluxes are being applied in liquid form or as powders or pastes. | Since the residues of fluxes are hygroscopic and can cause corrosion they have to be removed completely after the brazing process in very hot or boiling water. Depending on the type and process used, fluxes are being applied in liquid form or as powders or pastes. | ||
| − | <figtable id="tab: | + | <figtable id="tab:Fluxes for the Brazing of Heavy Metals"> |
'''Table 4.2: Fluxes for the Brazing of Heavy Metals''' | '''Table 4.2: Fluxes for the Brazing of Heavy Metals''' | ||
Revision as of 10:15, 30 April 2014
Contents
Brazing Alloys
For the joining of contact materials with carrier substrates brazing alloys with working temperatures > 600 °C are used exclusively. The working temperature is defined as the lowest surface temperature by which the brazing material wets the materials to be joined. This temperature is within the melting range and between the solidus (temperature at which melting starts) and liquidus (temperature at complete liquid state) point of the brazing alloy. Silver-based brazing alloys have good electrical conductivity and a sufficient mechanical strength which allows a bonding process without significant changes in the 4 microstructure of the material to be joined.
For electrical contacts usually low-melting alloys with a minimum of 20 wt-% silver and additions of cadmium, zinc or tin to lower the melting point are used Figure 1 (Table 4.1). Because of the toxicity of cadmium most cadmium containing brazing alloys have been replaced by zinc and tin containing brazing alloys. For higher requirements on corrosion resistance or for easier wetting of stainless steel nickel and manganese containing alloys are also used. Using any of these brazing alloys in an air environment is only possible with the addition of oxide reducing fluxes.
For high temperature brazing in vacuum or protective atmosphere vacuum melted silver-copper eutectic brazing alloys are used. These also allow subsequent forming operations due to their higher ductility. For the brazing of contacts with a silver bottom layer to copper backings phosphorous containing brazing alloys which eliminate the need for a flux application are widely used. The brazing alloy is typically introduced into the joint area in the form of wire segments, foil, shims, or as powder or paste. For larger production volumes it is economically advantageous to pre-coat contact tips with a thin layer (≤ 100 µm) of brazing alloy.
Fluxes
Brazing fluxes consist of non-metallic materials, mostly salt mixtures of boron and halogen compounds Table 1 (Table 4.2). Their purpose is to remove oxides from the brazing surfaces and prevent their new build-up in order to allow a thorough wetting of these surfaces by the liquefied brazing alloy. Fluxes have to be activated already at a temperature below the working range of the brazing alloy. They are selected mainly according to the working temperature of the brazing alloy and the base material to be joined.
Since the residues of fluxes are hygroscopic and can cause corrosion they have to be removed completely after the brazing process in very hot or boiling water. Depending on the type and process used, fluxes are being applied in liquid form or as powders or pastes.
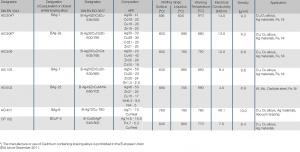
Designation DIN EN 1045 | Designation US (similar) | Active tempe- rature range [°C] | Chemical ingredients | Base materials used for |
|---|---|---|---|---|
TYP FH 10 | FB 3-A | 550 - 800 | Boron compounds, Fluorides | All metals and alloys except light metals, alloyed steels, carbide steels |
TYP FH 11 | FB 4-A | 550 - 800 | Boron compounds, Fluorides, Chlorides | Copper, Aluminum bronze |
TYP FH 12 | FB 3-C | 550 - 850 | Boron, Boron compounds, Fluorides | Special brass, any steel alloys, carbide steel |
TYP FH 21 | FB 3-I | 750 - 1100 | Boron compounds, Chlorides | All metals and alloys except light metals |
References
DIN 8514 Löten metallischer Werkstoffe. Begriffe, Benennungen
DIN EN 1044 Hartlöten, Lötzusätze
DIN EN 1045 Flussmittel zum Hartlöten
ASM Metals, Handbook: Vol 6 Welding, Brazing, and Soldering, ASM, Cleveland, OH, 1993
Dorn, L.: Hartlöten, Grundlagen und Anwendungen. Expert-Verlag, Band 146 (1985)
Krell, A.: Flussmittel und Lötfehler beim Hartlöten. Schweißtechnik, Berlin, 38 (1988)
DVS-Taschenbuch 196: Beuth-Verlag. Berlin, 1997
Müller, W.: Metallische Lotwerkstoffe. Dt. Verlag für Schweißtechnik, Düsseldorf 1990