Difference between revisions of "Silver Based Materials"
(→Hard-Silver Alloys) |
(→Hard-Silver Alloys) |
||
| Line 348: | Line 348: | ||
Dispersion hardened alloys of silver with 0.5 wt% MgO and NiO (ARGODUR 32) are produced by internal oxidation. While the melt-metallurgical alloy is easy to cold-work and form the material becomes very hard and brittle after dispersion hardening. Compared to fine silver and hard-silver this material has a greatly improved temperature stability and can be exposed to brazing temperatures up to 800°C without decreasing its hardness and tensile strength. | Dispersion hardened alloys of silver with 0.5 wt% MgO and NiO (ARGODUR 32) are produced by internal oxidation. While the melt-metallurgical alloy is easy to cold-work and form the material becomes very hard and brittle after dispersion hardening. Compared to fine silver and hard-silver this material has a greatly improved temperature stability and can be exposed to brazing temperatures up to 800°C without decreasing its hardness and tensile strength. | ||
| − | Because of these mechanical properties and its high electrical conductivity | + | Because of these mechanical properties and its high electrical conductivity ARGODUR 32 is mainly used in the form of contact springs that are exposed to high thermal and mechanical stresses in relays, and contactors for aeronautic applications. |
| Line 355: | Line 355: | ||
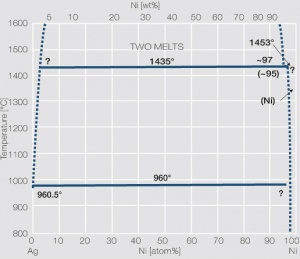
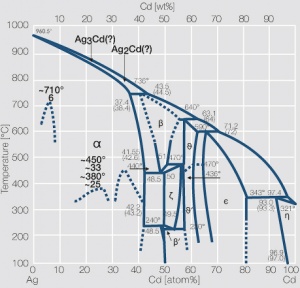
<xr id="fig:Phase diagram of silver cadmium"/> Fig. 2.53: Phase diagram of silver-cadmium | <xr id="fig:Phase diagram of silver cadmium"/> Fig. 2.53: Phase diagram of silver-cadmium | ||
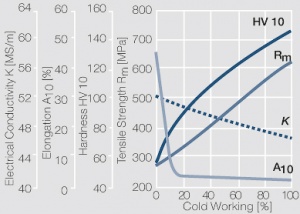
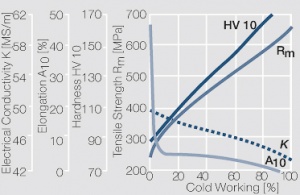
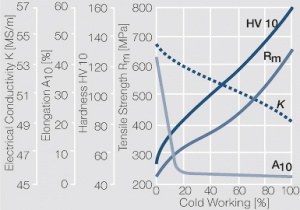
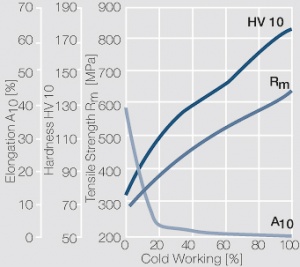
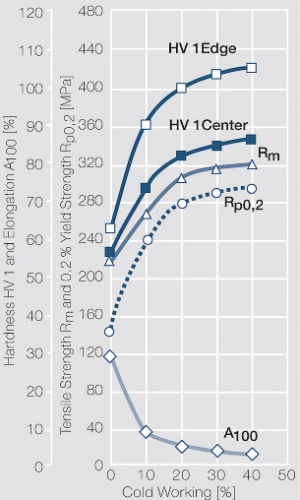
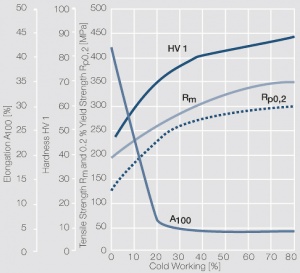
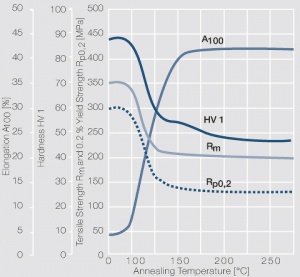
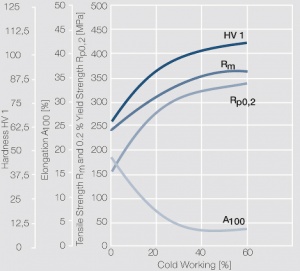
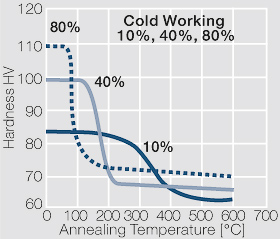
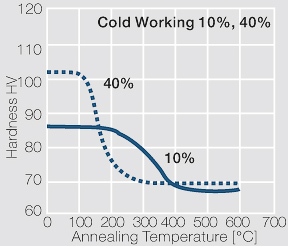
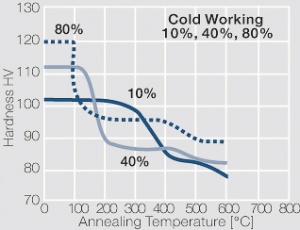
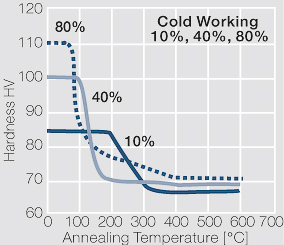
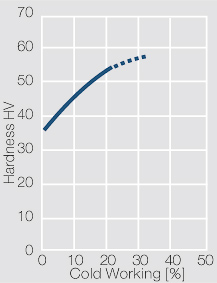
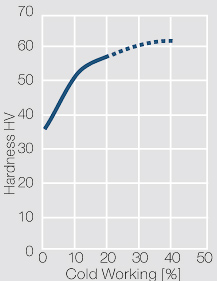
| − | <xr id="fig: | + | <xr id="fig:Strain hardening of AgCu3 by cold working"/> Fig. 2.54: Strain hardening of AgCu3 by cold working |
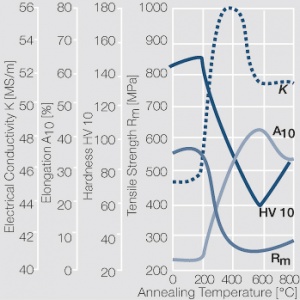
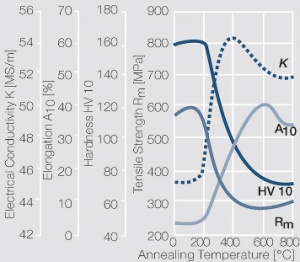
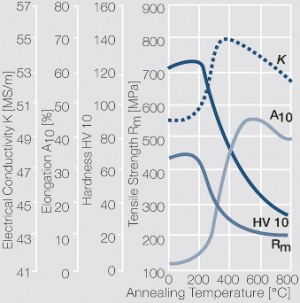
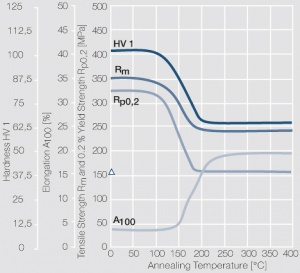
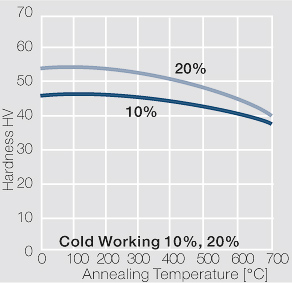
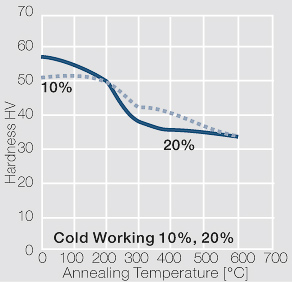
| − | <xr id="fig: | + | <xr id="fig:Softening of AgCu3 after annealing"/> Fig. 2.55: Softening of AgCu3 after annealing for 1 hr after 80% cold working |
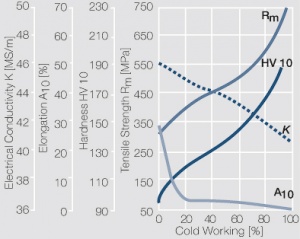
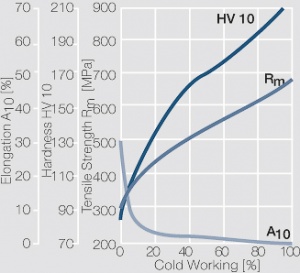
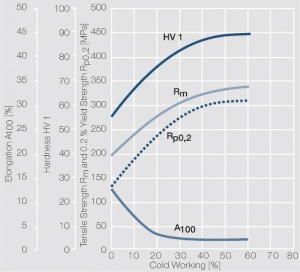
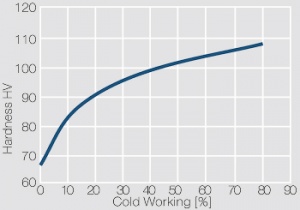
| − | <xr id="fig: | + | <xr id="fig:Strain hardening of AgCu5 by cold working"/> Fig. 2.56: Strain hardening of AgCu5 by cold working |
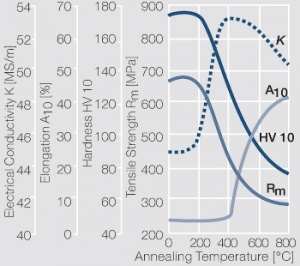
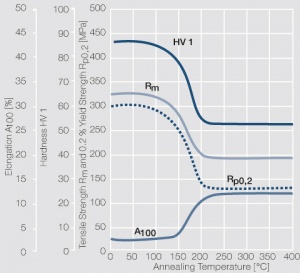
| − | <xr id="fig: | + | <xr id="fig:Softening of AgCu5 after annealing"/> Fig. 2.57: Softening of AgCu5 after annealing for 1 hr after 80% cold working |
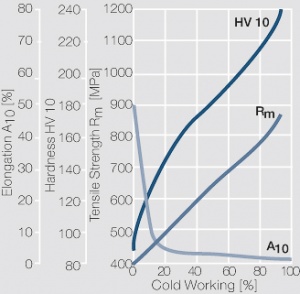
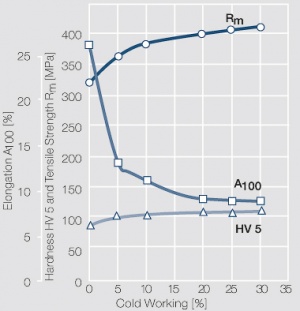
| − | <xr id="fig: | + | <xr id="fig:Strain hardening of AgCu 10 by cold working"/> Fig. 2.58: Strain hardening of AgCu 10 by cold working |
<xr id="fig:fig2.59"/> Fig. 2.59: Softening of AgCu10 after annealing for 1 hr after 80% cold working | <xr id="fig:fig2.59"/> Fig. 2.59: Softening of AgCu10 after annealing for 1 hr after 80% cold working | ||
| Line 389: | Line 389: | ||
</figure> | </figure> | ||
| − | <figure id="fig: | + | <figure id="fig:Strain hardening of AgCu3 by cold working"> |
[[File:Strain hardening of AgCu3 by cold working.jpg|left|thumb|<caption>Strain hardening of AgCu3 by cold working</caption>]] | [[File:Strain hardening of AgCu3 by cold working.jpg|left|thumb|<caption>Strain hardening of AgCu3 by cold working</caption>]] | ||
</figure> | </figure> | ||
| − | <figure id="fig: | + | <figure id="fig:Softening of AgCu3 after annealing"> |
[[File:Softening of AgCu3 after annealing.jpg|left|thumb|<caption>Softening of AgCu3 after annealing for 1 hr after 80% cold working</caption>]] | [[File:Softening of AgCu3 after annealing.jpg|left|thumb|<caption>Softening of AgCu3 after annealing for 1 hr after 80% cold working</caption>]] | ||
</figure> | </figure> | ||
| − | <figure id="fig: | + | <figure id="fig:Strain hardening of AgCu5 by cold working"> |
[[File:Strain hardening of AgCu5 by cold working.jpg|left|thumb|<caption>Strain hardening of AgCu5 by cold working</caption>]] | [[File:Strain hardening of AgCu5 by cold working.jpg|left|thumb|<caption>Strain hardening of AgCu5 by cold working</caption>]] | ||
</figure> | </figure> | ||
| − | <figure id="fig: | + | <figure id="fig:Softening of AgCu5 after annealing"> |
[[File:Softening of AgCu5 after annealing.jpg|left|thumb|<caption>Softening of AgCu5 after annealing for 1 hr after 80% cold working</caption>]] | [[File:Softening of AgCu5 after annealing.jpg|left|thumb|<caption>Softening of AgCu5 after annealing for 1 hr after 80% cold working</caption>]] | ||
</figure> | </figure> | ||
| − | <figure id="fig: | + | <figure id="fig:Strain hardening of AgCu 10 by cold working"> |
[[File:Strain hardening of AgCu 10 by cold working.jpg|left|thumb|<caption>Strain hardening of AgCu 10 by cold working</caption>]] | [[File:Strain hardening of AgCu 10 by cold working.jpg|left|thumb|<caption>Strain hardening of AgCu 10 by cold working</caption>]] | ||
</figure> | </figure> | ||
Revision as of 14:46, 28 April 2014
Contents
Pure Silver
Pure silver (also called fine silver) exhibits the highest electrical and thermal conductivity of all metals. It is also resistant against oxidation. Major disadvantages are its low mechanical wear resistance, the low softening temperature, and especially its strong affinity to sulfur and sulfur compounds. In the presence of sulfur and sulfur containing compounds brownish to black silver sulfide layer are formed on its surface. These can cause increased contact resistance or even total failure of a switching device if they are not mechanically, electrically, or thermally destroyed. Other weaknesses of silver contacts are the tendency to weld under the influence of over-currents and the low resistance against material transfer when switching DC loads. In humid environments and under the influence of an electrical field silver can creep (silver migration) and cause electrical shorting between adjacent current paths.
Table 1 (Table 2.11) shows the typically available quality grades of silver. In certain economic areas, i.e. China, there are additional grades with varying amounts of impurities available on the market. In powder form silver is used for a wide variety of silver based composite contact materials. Different manufacturing processes result in different grades of Ag powder as shown in Table 2 Table 2.12. additional properties of silver powders and their usage are described in Precious Metal Powders und Tab. 8.1 (Tab. 8.1.) Semi-finished silver materials can easily be warm or cold formed and can be clad to the usual base materials. For attachment of silver to contact carrier materials welding of wire or profile cut-offs and brazing are most widely applied. Besides these mechanical processes such as wire insertion (wire staking) and the riveting (staking) of solid or composite contact rivets are used in the manufacture of contact components.
Contacts made from fine silver are applied in various electrical switching devices such as relays, pushbuttons, appliance and control switches for currents < 2 A ??? (Table 2.16). Electroplated silver coatings are widely used to reduce the contact resistance and improve the brazing behavior of other contact materials and components.
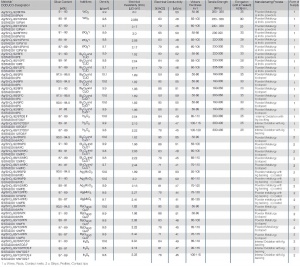
Designation | Composition minimum Ag [wt%] | Impurities [ppm] | Notes on Usage |
|---|---|---|---|
Spectroscopically Pure Ag | 99.999 | Cu < 3 Zn < 1 Si < 1 Ca < 2 Fe < 1 Mg < 1 Cd < 1 | Sheets, strips, rods, wires for electronic applications |
High Purity Ag, oxygen-free | 99.995 | Cu < 30 Zn < 2 Si < 5 Ca < 10 Fe < 3 Mg < 5 Cd < 3 | Ingots, bars, granulate for alloying purposes |
| Impurities | Ag-Chem.* | Ag-ES** | Ag-V*** | |
|---|---|---|---|---|
| Cu | ppm | < 100 | < 300 | < 300 |
| Fe | ppm | < 50 | < 100 | < 100 |
| Ni | ppm | < 50 | < 50 | < 50 |
| Cd | ppm | < 50 | ||
| Zn | ppm | < 10 | ||
| Na + K + Mg + Ca | ppm | < 80 | < 50 | < 50 |
| Ag CI | ppm | < 500 | < 500 | < 500 |
| NO3 | ppm | < 40 | < 40 | |
| Nh4CI | ppm | < 30 | < 30 | |
| Particle Size Distribution (screen analysis) | ||||
| > 100 μm | % | 0 | 0 | 0 |
| < 100 bis > 63 μm | % | < 5 | < 5 | < 15 |
| < 36 μm | % | < 80 | < 90 | < 75 |
| Apparent Density | g/cm3 | 1.0 - 1.6 | 1.0 - 1.5 | 3 - 4 |
| Tap Density | ml/100g | 40 - 50 | 40 - 50 | 15 - 25 |
| Press/Sintering Behavior | ||||
| Press Density | g/cm3 | 5.6 - 6.5 | 5.6 - 6.3 | 6.5 - 8.5 |
| Sinter Density | g/cm3 | > 9 | > 9.3 | > 8 |
| Volume Shrinkage | % | > 34 | > 35 | > 0 |
| Annealing Loss | % | < 2 | < 0.1 | < 0.1 |
* Manufactured by chemical precipitation
** Manufactured by electrolytic deposition
*** Manufactured by atomizing of a melt
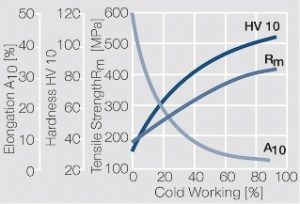
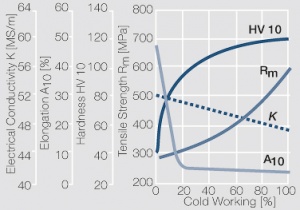
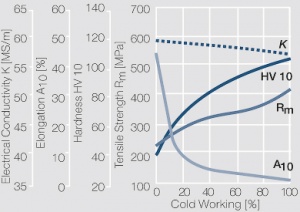
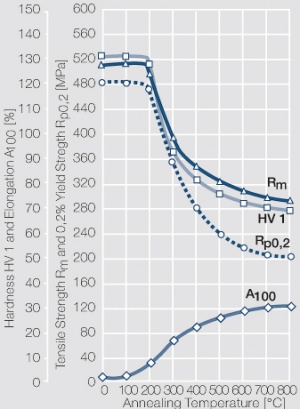
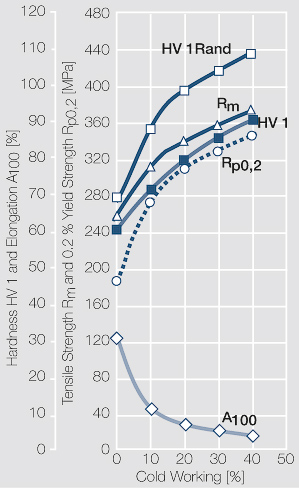
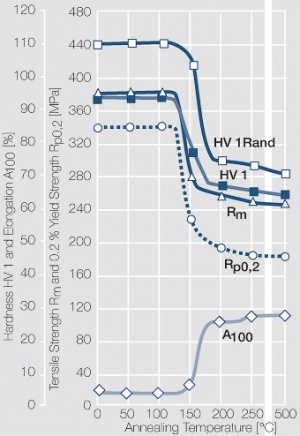
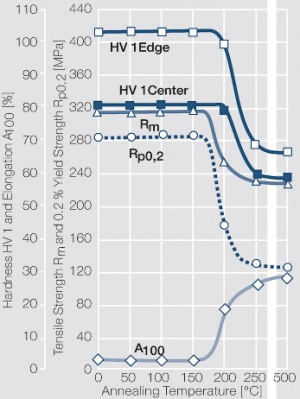
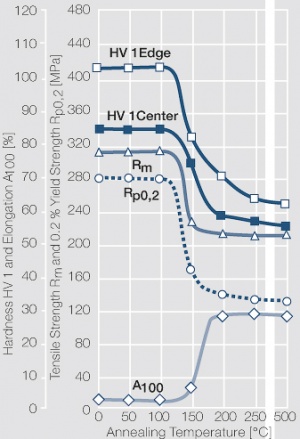
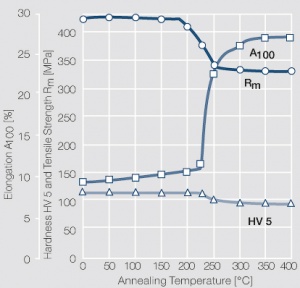
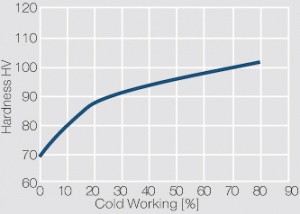
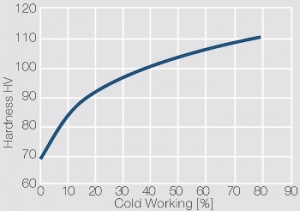
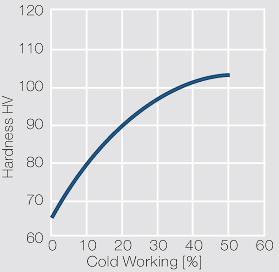
Figure 1 Fig. 2.45: Strain hardening of Ag 99.95 by cold working
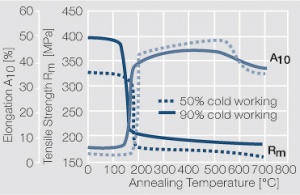
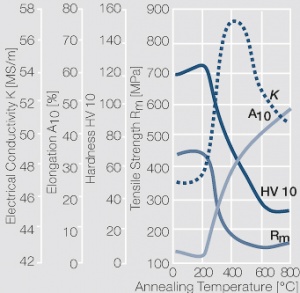
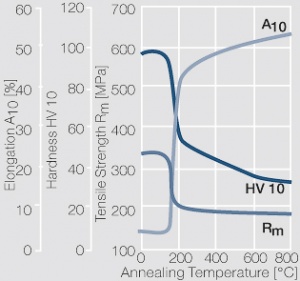
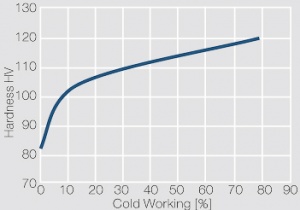
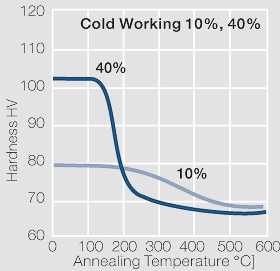
Figure 2 Fig. 2.46: Softening of Ag 99.95 after annealing for 1 hr after different degrees of strain hardening
Silver Alloys
To improve the physical and contact properties of fine silver melt-metallurgical produced silver alloys are used Table 3 (Table 2.13). By adding metal components the mechanical properties such as hardness and tensile strength as well as typical contact properties such as erosion resistance, and resistance against material transfer in DC circuits are increased ??? (Table 2.14). On the other hand however, other properties such as electrical conductivity and chemical corrosion resistance can be negatively impacted by alloying Figure 3 (Fig. 2.47) and Figure 4 (Fig. 2.48).
| Material/ DODUCO- Designation |
Silver Content [wt%] |
Density [g/cm3] |
Melting Point or Range [°C] |
Electrical Resistivity [μΩ·cm] |
Electrical Conductivity [MS/m] |
Thermal Conductivity [W/mK] |
Temp. Coefficient of the Electr.Resistance [10-3/K] |
Modulus of Elasticity [GPa] |
|---|---|---|---|---|---|---|---|---|
| Ag | 99.95 | 10.5 | 961 | 1.67 | 60 | 419 | 4.1 | 80 |
| AgNi 0,15 ARGODUR-Spezial |
99.85 | 10.5 | 960 | 1.72 | 58 | 414 | 4.0 | 82 |
| AgCu3 | 97 | 10.4 | 900 - 938 | 1.92 | 52 | 385 | 3.2 | 85 |
| AgCu5 | 95 | 10.4 | 910 | 1.96 | 51 | 380 | 3.0 | 85 |
| AgCu10 | 90 | 10.3 | 870 | 2.0 | 50 | 335 | 2.8 | 85 |
| AgCu28 | 72 | 10.0 | 779 | 2.08 | 48 | 325 | 2.7 | 92 |
| Ag98CuNi ARGODUR 27 |
98 | 10.4 | 940 | 1.92 | 52 | 385 | 3.5 | 85 |
| AgCu24,5Ni0,5 | 75 | 10.0 | 805 | 2.20 | 45 | 330 | 2.7 | 92 |
| AgCd10 | 89 - 91 | 10.3 | 910 - 925 | 4.35 | 23 | 150 | 1.4 | 60 |
| Ag99,5NiMg ARGODUR 32 Not heat treated |
99.5 | 10.5 | 960 | 2.32 | 43 | 293 | 2.3 | 80 |
| ARGODUR 32 Heat treated |
99.5 | 10.5 | 960 | 2.32 | 43 | 293 | 2.1 | 80 |
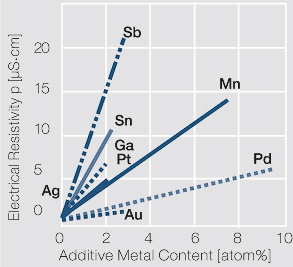
Figure 3 Fig. 2.47: Influence of 1-10 atom% of different alloying metals on the electrical resistivity of silver
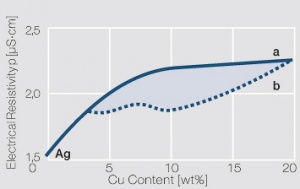
Figure 4 Fig. 2.48: Electrical resistivity p of AgCu alloys
Fine-Grain Silver
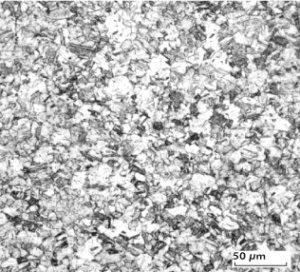
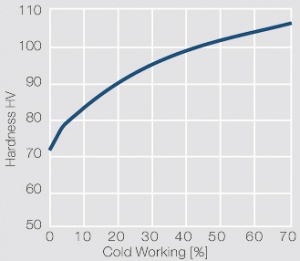
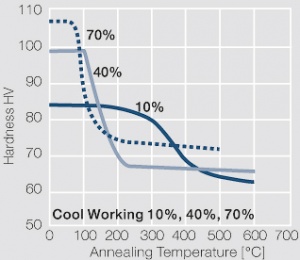
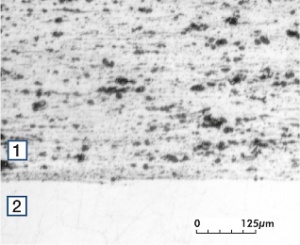
Fine-Grain Silver (ARGODUR-Spezial) is defined as a silver alloy with an addition of 0.15 wt% of Nickel. Silver and nickel are not soluble in each other in solid form. In liquid silver only a small amount of nickel is soluble as the phase diagram Figure 7 (Fig. 2.51) illustrates. During solidification of the melt this nickel addition gets finely dispersed in the silver matrix and eliminates the pronounce coarse grain growth after prolonged influence of elevated temperatures Figure 5 (Fig. 2.49) and Figure 6 (Fig. 2.50).
Fine-grain silver has almost the same chemical corrosion resistance as fine silver. Compared to pure silver it exhibits a slightly increased hardness and tensile strength Table 4 (Table 2.14). The electrical conductivity is just slightly decreased by this low nickel addition. Because of its significantly improved contact properties fine grain silver has replaced pure silver in many applications.
Hard-Silver Alloys
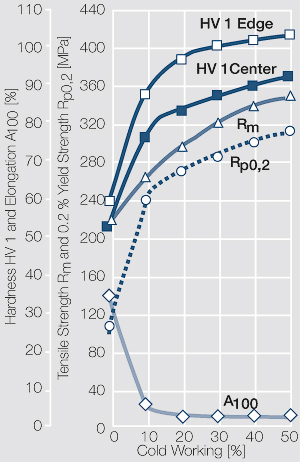
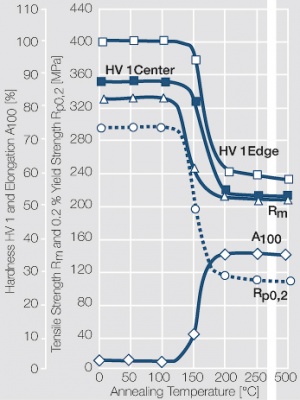
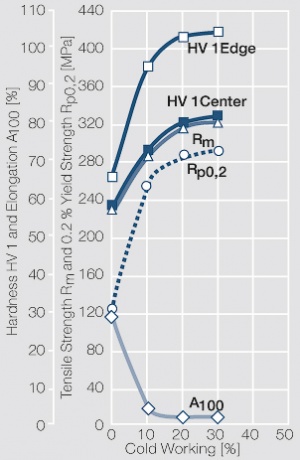
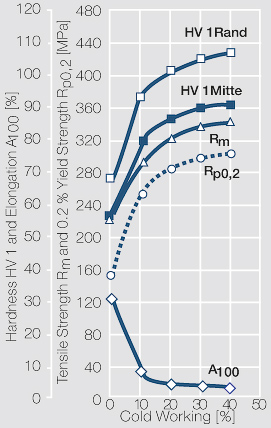
Using copper as an alloying component increases the mechanical stability of silver significantly. The most important among the binary AgCu alloys is that of AgCu3, known in europe also under the name of hard-silver. This material still has a chemical corrosion resistance close to that of fine silver. In comparison to pure silver and fine-grain silver AgCu3 exhibits increased mechanical strength as well as higher arc erosion resistance and mechanical wear resistance Table 4 (Table 2.14).
Material/ DODUCO-Designation | Hardness Condition | Tensile Strength Rm [MPa] | Elongation A [%] min. | Vickers Hardness HV 10 |
|---|---|---|---|---|
Ag | R 200 R 250 R 300 R 360 | 200 - 250 250 - 300 300 - 360 > 360 | 30 8 3 2 | 30 60 80 90 |
AgNi 0,15 ARGODUR Special | R 220 R 270 R 320 R 360 | 220 - 270 270 - 320 320 - 360 > 360 | 25 6 2 1 | 40 70 85 100 |
AgCu3 | R 250 R 330 R 400 R 470 | 250 - 330 330 - 400 400 - 470 > 470 | 25 4 2 1 | 45 90 115 120 |
AgCu5 | R 270 R 350 R 460 R 550 | 270 - 350 350 - 460 460 - 550 > 550 | 20 4 2 1 | 55 90 115 135 |
AgCu10 | R 280 R 370 R 470 R 570 | 280 - 370 370 - 470 470 - 570 > 570 | 15 3 2 1 | 60 95 130 150 |
AgCu28 | R 300 R 380 R 500 R 650 | 300 - 380 380 - 500 500 - 650 > 650 | 10 3 2 1 | 90 120 140 160 |
Ag98CuNi ARGODUR 27 | R 250 R 310 R 400 R 450 | 250 - 310 310 - 400 400 - 450 > 450 | 20 5 2 1 | 50 85 110 120 |
AgCu24,5Ni0,5 | R 300 R 600 | 300 - 380 > 600 | 10 1 | 105 180 |
AgCd10 | R 200 R 280 R 400 R 450 | 200 - 280 280 - 400 400 - 450 > 450 | 15 3 2 1 | 36 75 100 115 |
Ag99,5NiMg ARGODUR 32 Not heat treated | R 220 R 260 R 310 R 360 | 220 260 310 360 | 25 5 2 1 | 40 70 85 100 |
ARGODUR 32 Heat treated | R 400 | 400 | 2 | 130-170 |
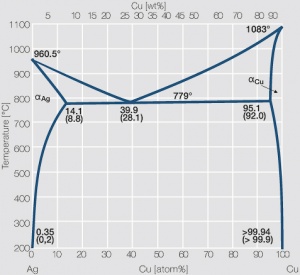
Increasing the Cu content further also increases the mechanical strength of AgCu alloys and improves arc erosion resistance and resistance against material transfer while at the same time however the tendency to oxide formation becomes detrimental. This causes during switching under arcing conditions an increase in contact resistance with rising numbers of operation. In special applications where highest mechanical strength is recommended and a reduced chemical resistance can be tolerated, the eutectic AgCu alloy with 28 wt% of copper Figure 8 (Fig. 2.52) is used. AgCu10 also known as coin silver has been replaced in many applications by composite silver-based materials while sterling silver (AgCu7.5) has never extended its important usage from decorative table wear and jewelry to industrial applications in electrical contacts.
Besides these binary alloys, ternary AgCuNi alloys are used in electrical contact applications. From this group the material ARGODUR 27, an alloy of 98 wt% Ag with a 2 wt% Cu and nickel addition has found practical importance close to that of AgCu3. This material is characterized by high resistance to oxidation and low tendency to re-crystallization during exposure to high temperatures. Besides high mechanical stability this AgCuNi alloy also exhibits a strong resistance against arc erosion. Because of its high resistance against material transfer the alloy AgCu24.5Ni0.5 has been used in the automotive industry for an extended time in the North American market. Caused by miniaturization and the related reduction in available contact forces in relays and switches this material has been replaced widely because of its tendency to oxide formation.
The attachment methods used for the hard silver materials are mostly close to those applied for fine silver and fine grain silver.
Hard-silver alloys are widely used for switching applications in the information and energy technology for currents up to 10 A, in special cases also for higher current ranges Table 5 (Table 2.16).
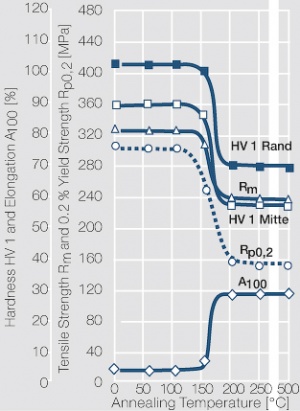
Dispersion hardened alloys of silver with 0.5 wt% MgO and NiO (ARGODUR 32) are produced by internal oxidation. While the melt-metallurgical alloy is easy to cold-work and form the material becomes very hard and brittle after dispersion hardening. Compared to fine silver and hard-silver this material has a greatly improved temperature stability and can be exposed to brazing temperatures up to 800°C without decreasing its hardness and tensile strength. Because of these mechanical properties and its high electrical conductivity ARGODUR 32 is mainly used in the form of contact springs that are exposed to high thermal and mechanical stresses in relays, and contactors for aeronautic applications.
Figure 8 Fig. 2.52: Phase diagram of silver-copper
Figure 9 Fig. 2.53: Phase diagram of silver-cadmium
Figure 10 Fig. 2.54: Strain hardening of AgCu3 by cold working
Figure 11 Fig. 2.55: Softening of AgCu3 after annealing for 1 hr after 80% cold working
Figure 12 Fig. 2.56: Strain hardening of AgCu5 by cold working
Figure 13 Fig. 2.57: Softening of AgCu5 after annealing for 1 hr after 80% cold working
Figure 14 Fig. 2.58: Strain hardening of AgCu 10 by cold working
Figure 15 Fig. 2.59: Softening of AgCu10 after annealing for 1 hr after 80% cold working
Figure 16 Fig. 2.60: Strain hardening of AgCu28 by cold working
Figure 17 Fig. 2.61: Softening of AgCu28 after annealing for 1 hr after 80% cold working
Figure 18 Fig. 2.62: Strain hardening of AgNi0.15 by cold working
Figure 19 Fig. 2.63: Softening of AgNi0.15 after annealing for 1 hr after 80% cold working
Figure 20 Fig. 2.64: Strain hardening of ARGODUR 27 by cold working
Figure 21 Fig. 2.65: Softening of ARGODUR 27 after annealing for 1 hr after 80% cold working
Table 2.15: Contact and Switching Properties of Silver and Silver Alloys
| Material | Properties | |
|---|---|---|
| Ag AgNi0,15 ARGODUR-Special |
Highest electrical and thermal conductivity, high affinity to sulfur (sulfide formation), low welding resistance, low contact resistance, very good formability | Oxidation resistant at higher make currents, limited arc erosion resistance, tendency to material transfer in DC circuits, easy to braze and weld to carrier materials |
| Ag Alloys | Increasing contact resistance with increasing
Cu content, compared to fine Ag higher arc erosion resistance and mechanical strength, lower tendency to material |
Good formability, good brazing and welding properties |
| Material | Application Examples | Form of Supply |
|---|---|---|
| Ag AgNi0,15 ARGODUR-Spezial AgCu3 AgNi98NiCu2 ARGODUR 27 AgCu24,5Ni0,5 |
Relays, Micro switches, Auxiliary current switches, Control circuit devices, Appliance switches, Wiring devices (< 20A), Main switches |
Semi-finished Materials: Strips, wires, contact profiles, clad contact strips, toplay profiles, seam- welded strips Contact Parts: Contact tips, solid and composite rivets, weld buttons; clad, welded and riveted contact parts |
| AgCu5 AgCu10 AgCu28 |
Special applications | Semi-finished Materials: Strips, wires, contact profiles, clad contact strips, seam-welded strips Contact parts: Contact tips, solid contact rivets, weld buttons; clad, welded and riveted contact parts |
| Ag99, 5NiOMgO ARGODUR 32 |
Miniature relays, aerospace relays and contactors, erosion wire for injection nozzles | Contact springs, contact carrier parts |
Silver-Palladium Alloys
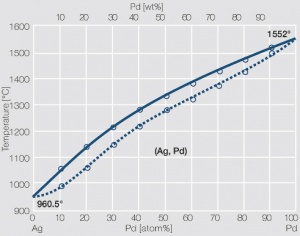
The addition of 30 wt% Pd increases the mechanical properties as well as the resistance of silver against the influence of sulfur and sulfur containing compounds significantly Table 6 (Tab 2.17) and Table 7 (Tab.2.18). Alloys with 40-60 wt% Pd have an even higher resistance against silver sulfide formation. At these percentage ranges however the catalytic properties of palladium can influence the contact resistance behavior negatively. The formability also decreases with increasing Pd contents.
AgPd alloys are hard, arc erosion resistant, and have a lower tendency towards material transfer under DC loads Table 8 (Table 2.19). On the other hand the electrical conductivity is decreased at higher Pd contents. The ternary alloy AgPd30Cu5 has an even higher hardness which makes it suitable for use in sliding contact systems.
AgPd alloys are mostly used in relays for the switching of medium to higher loads (>60V, >2A) as shown in Table 9 (Table 2.20). Because of the high palladium price these formerly solid contacts have been widely replaced by multi-layer designs such as AgNi0.15 or AgNi10 with a thin Au surface layer. A broader field of application for AgPd alloys remains in the wear resistant sliding contact systems.
Figure 22 Fig. 2.66: Phase diagram of silver-palladium
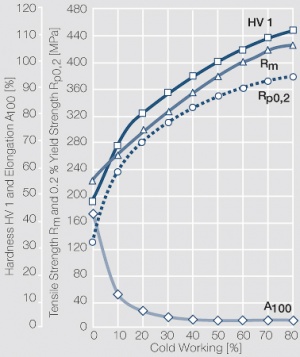
Figure 23 Fig. 2.67: Strain hardening of AgPd30 by cold working
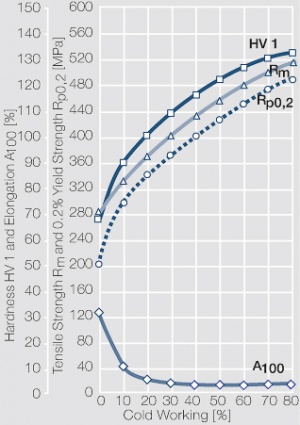
Figure 24 Fig. 2.68: Strain hardening of AgPd50 by cold working
Figure 25 Fig. 2.69: Strain hardening of AgPd30Cu5 by cold working
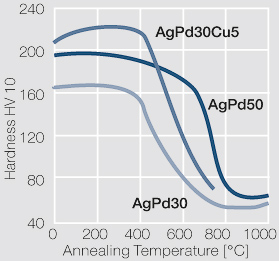
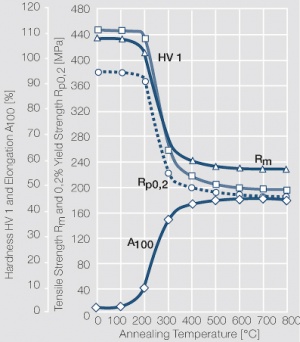
Figure 26 Fig. 2.70: Softening of AgPd30, AgPd50, and AgPd30Cu5 after annealing of 1 hr after 80% cold working
| Material | Palladium Content [wt%] |
Density [g/cm3] |
Melting Point or Range [°C] |
Electrical Resistivity [μΩ·cm] |
Electrical Conductivity [MS/m] |
Thermal Conductivity [W/m·K] |
Temp. Coefficient of the Electr.Resistance [10-3/K] |
|---|---|---|---|---|---|---|---|
| AgPd30 | 30 | 10.9 | 1155 - 1220 | 14.7 | 6.8 | 60 | 0.4 |
| AgPd40 | 40 | 11.1 | 1225 - 1285 | 20.8 | 4.8 | 46 | 0.36 |
| AgPd50 | 50 | 11.2 | 1290 - 1340 | 32.3 | 3.1 | 34 | 0.23 |
| AgPd60 | 60 | 11.4 | 1330 - 1385 | 41.7 | 2.4 | 29 | 0.12 |
| AgPd30Cu5 | 30 | 10.8 | 1120 - 1165 | 15.6 | 6.4 | 28 | 0.37 |
Material | Hardness Condition | Tensile Strength Rm[MPa] | Elongation A [%]min. | Vickers Hardness HV |
|---|---|---|---|---|
AgPd30 | R 320 R 570 | 320 570 | 38 3 | 65 145 |
AgPd40 | R 350 R 630 | 350 630 | 38 2 | 72 165 |
AgPd50 | R 340 R 630 | 340 630 | 35 2 | 78 185 |
AgPd60 | R 430 R 700 | 430 700 | 30 2 | 85 195 |
AgPd30Cu5 | R 410 R 620 | 410 620 | 40 2 | 90 190 |
| Material | Properties | |
|---|---|---|
| AgPd30-60 | Corrosion resistant, tendency to Brown Powder formation increases with Pd content, low tendency to material transfer in DC circuits, high ductility | Resistant against Ag2S formation, low contact resistance, increasing hardness with higher Pd content, AgPd30 has highest arc erosion resistance, easy to weld and clad |
| AgPd30Cu5 | High mechanical wear resistance | High Hardness |
Material | Application Examples | Form of Supply |
|---|---|---|
AgPd 30-60 | Switches, relays, push-buttons, connectors, sliding contacts | Semi-finished Materials: Wires, micro profiles (weld tapes), clad contact strips, seam-welded strips Contact Parts: Solid and composite rivets, weld buttons; clad and welded contact parts, stamped parts |
AgPd30Cu5 | Sliding contacts, slider tracks | Wire-formed parts, contact springs, solid and clad stamped parts |
Silver Composite Materials
Silver-Nickel (SINIDUR) Materials
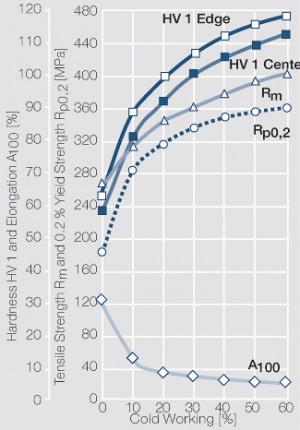
Since silver and nickel are not soluble in each other in solid form and in the liquid phase have only very limited solubility silver nickel composite materials with higher Ni contents can only be produced by powder metallurgy. During extrusion of sintered Ag/Ni billets into wires, strips and rods the Ni particles embedded in the Ag matrix are stretched and oriented in the microstructure into a pronounced fiber structure Figure 31 (Fig. 2.75) and Figure 32 (Fig. 2.76)
The high density produced during hot extrusion aids the arc erosion resistance of these materials Table 10 (Tab 2.21). The typical application of Ag/Ni contact materials is in devices for switching currents of up to 100A Table 13 (Table 2.24). In this range they are significantly more erosion resistant than silver or silver alloys. In addition they exhibit with nickel contents <20 wt% a low and over their operational lifetime consistent contact resistance and good arc moving properties. In DC applications Ag/Ni materials exhibit a relatively low tendency of material transfer distributed evenly over the contact surfaces Table 12 (Table 2.23).
Typically Ag/Ni (SINIDUR) materials are usually produced with contents of 10-40 wt% Ni. The most widely used materials SINIDUR 10 and SINIDUR 20- and also SINIDUR 15, mostly used in north america-, are easily formable and applied by cladding Figure 27 (Fig. 2.71) Figure 28 (Fig. 2.72) Figure 29 (Fig. 2.73) Figure 30 (Fig. 2.74). They can be, without any additional welding aids, economically welded and brazed to the commonly used contact carrier materials. The (SINIDUR) materials with nickel contents of 30 and 40 wt% are used in switching devices requiring a higher arc erosion resistance and where increases in contact resistance can be compensated through higher contact forces.
The most important applications for Ag/Ni contact materials are typically in relays, wiring devices, appliance switches, thermostatic controls, auxiliary switches, and small contactors with nominal currents >20A Table 13 (Table 2.24).
| Material/DODUCO | Silver Content | Density | Melting Point | ElectricalResistivityp | Electrical Resistivity (soft) | |
|---|---|---|---|---|---|---|
| Designation | [wt%] | [g/cm3] | [°C] | [µΩ·cm] | [% IACS] | [MS/m] |
Ag/Ni 90/10 SINIDUR 10 | 89 - 91 | 10.2 - 10.3 | 960 | 1.82 - 1.92 | 90 - 95 | 52 - 55 |
Ag/Ni 85/15 SINIDUR 15 | 84 - 86 | 10.1 - 10.2 | 960 | 1.89 - 2.0 | 86 - 91 | 50 - 53 |
Ag/Ni 80/20 SINIDUR 20 | 79 - 81 | 10.0 - 10.1 | 960 | 1.92 - 2.08 | 83 - 90 | 48 - 52 |
Ag/Ni 70/30 SINIDUR 30 | 69 - 71 | 9.8 | 960 | 2.44 | 71 | 41 |
Ag/Ni 60/40 SINIDUR 40 | 59 - 61 | 9.7 | 960 | 2.70 | 64 | 37 |
| Material/DODUCO-Designation | Hardness Condition | Tensile Strength Rm [Mpa] | Elongation A (soft annealed) [%] min. | Vickers Hardness HV 10 |
|---|---|---|---|---|
| Ag/Ni 90/10 SINIDUR 10 |
soft R 220 R 280 R 340 R 400 |
< 250 220 - 280 280 - 340 340 - 400 > 400 |
25 20 3 2 1 |
< 50 50 - 70 65 - 90 85 - 105 > 100 |
| Ag/Ni 85/15 SINIDUR 15 |
soft R 300 R 350 R 380 R 400 |
< 275 250 - 300 300 - 350 350 - 400 > 400 |
20 4 2 2 1 |
< 70 70 - 90 85 - 105 100 - 120 > 115 |
| Ag/Ni 80/20 SINIDUR 20 |
soft R 300 R 350 R 400 R 450 |
< 300 300 - 350 350 - 400 400 - 450 > 450 |
20 4 2 2 1 |
< 80 80 - 95 90 - 110 100 - 125 > 120 |
| Ag/Ni 70/30 SINIDUR 30 |
R 330 R 420 R 470 R 530 |
330 - 420 420 - 470 470 - 530 > 530 |
8 2 1 1 |
80 100 115 135 |
| Ag/Ni 60/40 SINIDUR 40 |
R 370 R 440 R 500 R 580 |
370 - 440 440 - 500 500 - 580 > 580 |
6 2 1 1 |
90 110 130 150 |
Figure 27 Fig. 2.71: Strain hardening of Ag/Ni 90/10 by cold working
Figure 28 Fig. 2.72: Softening of Ag/Ni 90/10 after annealing for 1 hr after 80% cold working
Figure 29 Fig. 2.73: Strain hardening of Ag/Ni 80/20 by cold working
Figure 30 Fig. 2.74: Softening of Ag/Ni 80/20 after annealing for 1 hr after 80% cold working
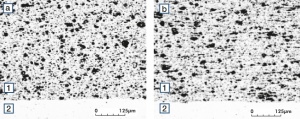
Figure 31 Fig. 2.75: Micro structure of Ag/Ni 90/10 a) perpendicular to the extrusion direction b) parallel to the extrusion direction
Figure 32 Fig. 2.76: Micro structure of Ag/Ni 80/20 a) perpendicular to the extrusion direction b) parallel t o the extrusion direction
| Material/DODUCO-Designation | Properties |
|---|---|
| Ag/Ni SINIDUR |
High arc erosion resistance at switching currents up to 100A, Resistance against welding for starting current up to 100A, low and over the electrical contact life nearly constant contact resistance for Ag/Ni 90/10 and Ag/Ni 80/20, ow and spread-out material transfer under DC load, non-conductive erosion residue on isolating components resulting in only minor change of the dielectric strength of switching devices, good arc moving properties, good arc extinguishing properties, good or sufficient ductility depending on the Ni content, easy to weld and braze |
| Material | Application Examples | Switching or Nominal Current | Form of Supply |
|---|---|---|---|
| Ag/Ni 90/10-80/20 | Relays ¤ Automotive Relays - Resistive load - Motor load |
> 10A > 10A |
Semi-finisched Materials: Wires, profiles, clad strips, Seam-welded strips, Toplay strips Contact Parts: Contact tips, solid and composite rivets, Weld buttons, clad, welded, brazed, and riveted contact parts |
| Ag/Ni 90/10, Ag/Ni 85/15-80/20 | Auxiliary current switches | ≤ 100A | |
| Ag/Ni 90/10-80/20 | Appliance switches | ≤ 50A | |
| Ag/Ni 90/10 | Wiring devices | ≤ 20A | |
| Ag/Ni 90/10 | Main switches, Automatic staircase illumination switches | ≤ 100A | |
| Ag/Ni 90/10-80/20 | Control Thermostats |
> 10A ≤ 50A | |
| Ag/Ni 90/10-80/20 | Load switches | ≤ 20A | |
| Ag/Ni 90/10-80/20 | Contactors circuit breakers | ≤ 100A | |
| Ag/Ni 90/10-80/20 paired with Ag/C 97/3-96/4 |
Motor protective circuit breakers | ≤ 40A | |
| Ag/Ni 80/20-60/40 paired with Ag/C 96/4-95/5 |
Fault current circuit breakers | ≤ 100A | Rods, Profiles, Contact tips, Formed parts, brazed and welded contact parts |
| Ag/Ni 80/20-60/40 paired with Ag/C 96/4-95/5 |
Power switches | > 100A |
Silver-Metal Oxide Materials Ag/CdO, Ag/SnO2, Ag/ZnO
The family of silver-metal oxide contact materials includes the material groups: silver-cadmium oxide (DODURIT CdO), silver-tin oxide (SISTADOX), and silverzinc oxide (DODURIT ZnO). Because of their very good contact and switching properties like high resistance against welding, low contact resistance, and high arc erosion resistance, silver-metal oxides have gained an outstanding position in a broad field of applications. They mainly are used in low voltage electrical switching devices like relays, installation and distribution switches, appliances, industrial controls, motor controls, and protective devices Table 18 (Table 2.31).
- Silver-cadmium oxide (DODURIT CdO) materials
Silver-cadmium oxide (DODURIT CdO) materials with 10-15 wt% are produced by both, internal oxidation and powder metallurgical methods Figure 33 (Table 2.25).
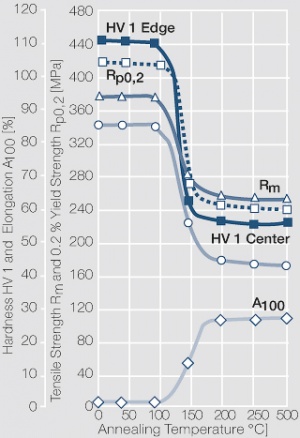
The manufacturing of strips and wires by internal oxidation starts with a molten alloy of silver and cadmium. During a heat treatment below it's melting point in a oxygen rich atmosphere in such a homogeneous alloy the oxygen diffuses from the surface into the bulk of the material and oxidizes the Cd to CdO in a more or less fine particle precipitation inside the Ag matrix. The CdO particles are rather fine in the surface area and are becoming larger further away towards the center of the material Figure 40 (Fig. 2.83).
During the manufacturing of Ag/CdO contact material by internal oxidation the processes vary depending on the type of semi-finished material. For Ag/CdO wires a complete oxidation of the AgCd wire is performed, followed by wire-drawing to the required diameter Figure 34 (Figs. 2.77) and Figure 35 (Fig. 2.78). The resulting material is used for example in the production of contact rivets. For Ag/CdO strip materials two processes are commonly used: Cladding of an AgCd alloy strip with fine silver followed by complete oxidation results in a strip material with a small depletion area in the center of it's thickness and a Ag backing suitable for easy attachment by brazing (sometimes called “Conventional Ag/CdO”). Using a technology that allows the partial oxidation of a dual-strip AgCd alloy material in a higher pressure pure oxygen atmosphere yields a composite Ag/CdO strip material that has besides a relatively fine CdO precipitation also a easily brazable AgCd alloy backing Figure 42 (Fig. 2.85). These materials (DODURIT CdO ZH) are mainly used as the basis for contact profiles and contact tips.
During powder metallurgical production the powder mixed made by different processes are typically converted by pressing, sintering and extrusion to wires and strips. The high degree of deformation during hot extrusion produces a uniform and fine dispersion of CdO particles in the Ag matrix while at the same time achieving a high density which is advantageous for good contact properties Figure 41 (Fig. 2.84). To obtain a backing suitable for brazing, a fine silver layer is applied by either com-pound extrusion or hot cladding prior to or right after the extrusion Figure 43 (Fig. 2.86).
For larger contact tips, and especially those with a rounded shape, the single tip Press-Sinter-Repress process (PSR) offers economical advantages. The powder mix is pressed in a die close to the final desired shape, the “green” tips are sintered, and in most cases the repress process forms the final exact shape while at the same time increasing the contact density and hardness.
Using different silver powders and minor additives for the basic Ag and CdO starting materials can help influence certain contact properties for specialized applications.
Figure 34 Fig. 2.77: Strain hardening of internally oxidized Ag/CdO 90/10 by cold working
Figure 35 Fig. 2.78: Softening of internally oxidized Ag/CdO 90/10 after annealing for 1 hr after 40% cold working
Figure 36 Fig. 2.79: Strain hardening of Ag/CdO 90/10 P by cold working
Figure 37 Fig. 2.80: Softening of Ag/CdO 90/10 P after annealing for 1 hr after 40% cold working
Figure 38 Fig. 2.81: Strain hardening of Ag/CdO 88/12 WP
Figure 39 Fig. 2.82: Softening of Ag/CdO 88/12WP after annealing for 1 hr after different degrees of cold working
Figure 40 Fig. 2.83: Micro structure of Ag/CdO 90/10 i.o. a) close to surface b) in center area
Figure 41 Fig. 2.84: Micro structure of Ag/CdO 90/10 P: a) perpendicular to extrusion direction b) parallel to extrusion direction
Figure 42 Fig. 2.85: Micro structure of Ag/CdO 90/10 ZH: 1) Ag/CdO layer 2) AgCd backing layer
Figure 43 Fig. 2.86: Micro structure of AgCdO 88/12 WP: a) perpendicular to extrusion direction b) parallel to extrusion direction
- Silver–tin oxide(SISTADOX)materials
Over the past years, many Ag/CdO contact materials have been replaced by Ag/SnO2 based materials with 2-14 wt% SnO2 because of the toxicity of Cadmium. This changeover was further favored by the fact that Ag/SnO2 contacts quite often show improved contact and switching properties such as lower arc erosion, higher weld resistance, and a significant lower tendency towards material transfer in DC switching circuits Table 17 (Table 2.30). Ag/SnO2 materials have been optimized for a broad range of applications by other metal oxide additives and modification in the manufacturing processes that result in different metallurgical, physical and electrical propertiesTable 15 (Tab. 2.28) und Table 16 (Table 2.29).
Manufacturing of Ag/SnO2 by internal oxidation is possible in principle, but during heat treatment of alloys containing > 5 wt% of tin in oxygen, dense oxide layers formed on the surface of the material prohibit the further diffusion of oxygen into the bulk of the material. By adding Indium or Bismuth to the alloy the internal oxidation is possible and results in materials that typically are rather hard and brittle and may show somewhat elevated contact resistance and is limited to applications in relays. To make a ductile material with fine oxide dispersion (SISTADOX TOS F) Figure 72 (Fig. 2.114) it is necessary to use special process variations in oxidation and extrusion which lead to materials with improved properties in relays. Adding a brazable fine silver layer to such materials results in a semifinished material suitable for the manufacture as smaller weld profiles (SISTADOX WTOS F) Figure 74 (Fig. 2.116). Because of their resistance to material transfer and low arc erosion these materials find for example a broader application in automotive relays Table 18 (Table 2.31).
Powder metallurgy plays a significant role in the manufacturing of Ag/SnO2 contact materials. Besides SnO2 a smaller amount (<1 wt%) of one or more other metal oxides such as WO3, MoO3, CuO and/or Bi2O3 are added. These additives improve the wettability of the oxide particles and increase the viscosity of the Ag melt. They also provide additional benefits to the mechanical and arcing contact properties of materials in this group Figure 44 (Table 2.26 als PDF herunterladen: File:Physical Mechanical properties.pdf ).
Table 2.26: Physical and Mechanical Properties as well as Manufacturing Processes and Forms of Supply of Extruded Silver-Tin Oxide (SISTADOX) Contact Materials
In the manufacture the initial powder mixes different processes are applied which provide specific advantages of the resulting materials in respect to their contact properties . Some of them are described here as follows:
- a) Powder blending from single component powders
In this common process all components including additives that are part of the powder mix are blended as single powders. The blending is usually performed in the dry stage in blenders of different design.
- b) Powder blending on the basis of doped powders
For incorporation of additive oxides in the SnO2 powder the reactive spray process (RSV) has shown advantages. This process starts with a waterbased solution of the tin and other metal compounds. This solution is nebulized under high pressure and temperature in a reactor chamber. Through the rapid evaporation of the water each small droplet is converted into a salt crystal and from there by oxidation into a tin oxide particle in which the additive metals are distributed evenly as oxides. The so created doped AgSnO2 powder is then mechanically mixed with silver powder.
- c) Powder blending based on coated oxide powders
In this process tin oxide powder is blended with lower meting additive oxides such as for example Ag2 MoO4 and then heat treated. The SnO2 particles are coated in this step with a thin layer of the additive oxide.
- d) Powder blending based on internally oxidized alloy powders
A combination of powder metallurgy and internal oxidation this process starts with atomized Ag alloy powder which is subsequently oxidized in pure oxygen. During this process the Sn and other metal components are transformed to metal oxide and precipitated inside the silver matrix of each powder particle.
- e) Powder blending based on chemically precipitated compound powders
A silver salt solution is added to a suspension of for example SnO2 together with a precipitation agent. In a chemical reaction silver and silver oxide respectively are precipitated around the additive metal oxide particles who act as crystallization sites. Further chemical treatment then reduces the silver oxide with the resulting precipitated powder being a mix of Ag and SnO2.
Further processing of these differently produced powders follows the conventional processes of pressing, sintering and hot extrusion to wires and strips. From these contact parts such as contact rivets and tips are manufactured. To obtain a brazable backing the same processes as used for Ag/CdO are applied. As for Ag/CdO, larger contact tips can also be manufactured more economically using the press-sinter-repress (PSR) process Table 14 (Table 2.27).
Figure 45 Fig. 2.87: Strain hardening of Ag/SnO2 92/8 PE by cold working
Figure 46 Fig. 2.88: Softening of Ag/SnO2 92/8 PE after annealing for 1 hr after 40% cold working
Figure 47 Fig. 2.89: Strain hardening of Ag/SnO2 88/12 PE by cold working
Figure 48 Fig. 2.90: Softening of Ag/SnO2 88/12 PE after annealing for 1 hr after 40% cold working
Figure 49 Fig. 2.91: Strain hardening of oxidized Ag/SnO2 88/12 PW4 by cold working
Figure 50 Fig. 2.92: Softening of Ag/SnO2 88/12 PW4 after annealing for 1 hr after 30% cold working
Figure 51 Fig. 2.93: Strain hardening of Ag/SnO2 98/2 PX by cold working
Figure 52 Fig. 2.94: Softening of Ag/SnO2 98/2 PX after annealing for 1 hr after 80% cold working
Figure 53 Fig 2.95: Strain hardening of Ag/SnO2 92/8 PX by cold working
Figure 54 Fig. 2.96: Softening of Ag/SnO2 92/8 PX after annealing for 1 hr after 40% cold working
Figure 55 Fig. 2.97: Strain hardening of internally oxidized Ag/SnO2 88/12 TOS F by cold working
Figure 56 Fig. 2.98: Softening of Ag/SnO2 88/12 TOS F after annealing for 1 hr after 30% cold working
Figure 57 Fig. 2.99: Strain hardening of internally oxidized Ag/SnO2 88/12P by cold working
Figure 58 Fig. 2.100: Softening of Ag/SnO2 88/12P after annealing for 1 hr after 40% cold working
Figure 59 Fig. 2.101: Strain hardening of Ag/SnO2 88/12 WPC by cold working
Figure 60 Fig. 2.102: Softening of Ag/SnO2 88/12 WPC after annealing for 1 hr after different degrees of cold working
Figure 61 Fig. 2.103: Strain hardening of Ag/SnO2 86/14 WPC by cold working
Figure 62 Fig. 2.104: Softening of Ag/SnO2 86/14 WPC after annealing for 1 hr after different degrees of cold working
Figure 63 Fig. 2.105: Strain hardening of Ag/SnO2 88/12 WPD by cold working
Figure 64 Fig. 2.106: Softening of Ag/SnO2 88/12 WPD after annealing for 1 hr after different degrees of cold working
Figure 65 Fig. 2.108: Softening of Ag/SnO2 88/12 WPX after annealing for 1 hr after different degrees of cold working
Figure 66 Fig. 2.107: Strain hardening of Ag/SnO2 88/12 WPX by cold working
Figure 67 Fig. 2.109: Micro structure of Ag/SnO2 92/8 PE: a) perpendicular to extrusion direction b) parallel to extrusion direction
Figure 68 Fig. 2.110: Micro structure of Ag/SnO2 88/12 PE: a) perpendicular to extrusion direction b) parallel to extrusion direction
Figure 69 Fig. 2.111: Micro structure of Ag/SnO2 88/12 PW: a) perpendicular to extrusion direction b) parallel to extrusion direction
Figure 70 Fig. 2.112: Micro structure of Ag/SnO2 98/2 PX: a) perpendicular to extrusion direction b) parallel to extrusion direction
Figure 71 Fig. 2.113: Micro structure of Ag/SnO2 92/8 PX: a) perpendicular to extrusion direction b) parallel to extrusion direction
Figure 72 Fig. 2.114: Micro structure of Ag/SnO2 88/12 TOS F: a) perpendicular to extrusion direction b) parallel to extrusion direction
Figure 73 Fig. 2.115: Micro structure of Ag/SnO2 86/14 WPC: a) perpendicular to extrusion direction b) parallel to extrusion direction, 1) AgSnO2 contact layer, 2) Ag backing layer
Figure 74 Fig. 2.116: Micro structure of Ag/SnO2 92/8 WTOS F: a) perpendicular to extrusion direction b) parallel to extrusion direction,1) AgSnO2 contact layer, 2) Ag backing layer
Figure 75 Fig. 2.117: Micro structure of Ag/SnO2 88/12 WPD: parallel to extrusion direction 1) AgSnO2 contact layer, 2) Ag backing layer
Figure 76 Fig. 2.118: Micro structure of Ag/SnO2 88/12 WPX:parallel to extrusion direction 1) AgSnO2 contact layer, 2) Ag backing layer
Figure 77 Fig. 2.119: Micro structure of Ag/SnO2 86/14 WPX: a) perpendicular to extrusion direction b) parallel to extrusion direction, 1) AgSnO2 contact layer, 2) Ag backing layer
Material/ DODUCO- Designation | Additives | Density [ g/cm3] | Electrical Resistivity [µS ·cm] | Electrical Conductivity | Vickers Hardness HV 10. | |
|---|---|---|---|---|---|---|
[%IACS] | [MS/m] | |||||
AgCdO 90/10EP DODURIT CdO 10EP | 10.1 | 2.08 | 83 | 48 | 60 | |
AgCdO 85/15 EP DODURIT CdO 15EP | 9.9 | 2.27 | 76 | 44 | 65 | |
AgSnO² 90/10 EPX SISTADOX 10EPX | CuO and Bi² O³ | 9.8 | 2.22 | 78 | 45 | 55 |
AgSnO² 88/12EPX SISTADOX 12EPX | CuO and Bi² O³ | 9.6 | 2.63 | 66 | 38 | 60 |
- Silver–zinc oxide (DODURIT ZnO) materials
Silver zinc oxide (DODURIT ZnO) contact materials with mostly 6 - 10 wt% oxide content including other small metal oxides are produced exclusively by powder metallurgy (Figs. 76 – 81), (Table 2.28). Adding Ag2WO4 in the process b) as described in the preceding chapter on Ag/SnO2 has proven most effective for applications in AC relays, wiring devices, and appliance controls. Just like with the other Ag metal oxide materials, semi-finished materials in strip and wire form are used to manufacture contact tips and rivets. Because of their high resistance against welding and arc erosion Ag/ZnO materials present an economic alternative to Cd free Ag-tin oxide contact materials Table 17 (Tab. 2.30) and Table 18 (Tab. 2.31).
| Material/ DODUCO- Designation |
Silver Content [wt%] |
Additives | Density [g/cm3] |
Electrical Resistivity [μΩ·cm] |
Electrical Conductivity [% IACS] [MS/m] |
Vickers Hardness Hv1 |
Tensile Strength [MPa] |
Elongation (soft annealed) A[%]min. |
Manufacturing Process |
Form of Supply | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag/ZnO 92/8P DODURIT ZnO 8P |
91 - 93 | 9.8 | 2.22 | 78 | 45 | 60 - 95 | 220 - 350 | 25 | Powder Metallurgy a) indiv. powders |
1 | |
| Ag/ZnO 94/6PW25 DODURIT ZnO 6PW25 |
93 - 95 | Ag2WO4 | 9.7 | 2.0 | 86 | 50 | 60 - 100 | 200 - 320 | 30 | Powder Metallurgy c) coated |
1 |
| Ag/ZnO 92/8PW25 DODURIT ZnO 8PW25 |
91 - 93 | Ag2WO4 | 9.6 | 2.08 | 83 | 48 | 65 - 105 | 230 - 340 | 25 | Powder Metallurgy c) coated |
1 |
| Ag/ZnO 90/10PW25 DODURIT ZnO 10PW25 |
89 - 91 | Ag2WO4 | 9.6 | 2.17 | 79 | 46 | 65 - 100 | 230 - 350 | 20 | Powder Metallurgy c) coated |
1 |
| Ag/ZnO 92/8WP DODURIT ZnO 8WP |
91 - 93 | 9.8 | 2.0 | 86 | 50 | 60 - 95 | Powder Metallurgy with Ag backing a) individ. |
2 | |||
| AgZnO 94/6WPW25 DODURIT ZnO 6WPW25 |
93 - 95 | Ag2WO4 | 9.7 | 2.0 | 86 | 50 | 60 - 95 | Powder Metallurgy c) coated |
2 | ||
| Ag/ZnO 92/8WPW25 DODURIT ZnO 8WPW25 |
91 - 93 | Ag2WO4 | 9.6 | 2.08 | 83 | 48 | 65 - 105 | Powder Metallurgy c) coated |
2 | ||
| Ag/ZnO 90/10WPW25 DODURIT ZnO 10WPW25 |
89 - 91 | Ag2WO4 | 9.6 | 2.7 | 79 | 46 | 65 - 110 | Powder Metallurgy c) coated |
2 | ||
1 = Wires, Rods, Contact rivets, 2 = Strips, Profiles, Contact tips
Figure 78 Fig. 2.120: Strain hardening of Ag/ZnO 92/8 PW25 by cold working
Figure 79 Fig. 2.121: Softening of Ag/ZnO 92/8 PW25 after annealing for 1 hr after 30% cold working
Figure 80 Fig. 2.122: Strain hardening of Ag/ZnO 92/8 WPW25 by cold working
Figure 81 Fig. 2.123: Softening of Ag/ZnO 92/8 WPW25 after annealing for 1hr after different degrees of cold working
Figure 82 Fig. 2.124: Micro structure of Ag/ZnO 92/8 Pw25: a) perpendicular to extrusion direction b) parallel to extrusion direction
Figure 83 Fig. 2.125: Micro structure of Ag/ZnO 92/8 WPW25:a) perpendicular to extrusion direction b) parallel to extrusion direction, 1) Ag/ZnO contact layer, 2) Ag backing layer
Material/ Material Group | Special Properties | ||
|---|---|---|---|
Ag/SnO2 PE | Especially suitable for automotive relays (lamp loads) | Good formability (contact rivets) | |
Ag/SnO2 98/2 PX/PC | Especially good heat resistance | Easily riveted, can be directly welded | |
Ag/SnO2 TOS F | Especially suited for high inductive DC loads | Very good formability (contact rivets) | |
Ag/SnO2 WPC | For AC-3 and AC-4 applications in motor switches (contactors) | ||
Ag/SnO2 WPD | Especially suited for severe loads (AC-4) and high switching currents | ||
Ag/SnO2 WPX | For standard motor loads (AC-3) and Resistive loads (AC-1), DC loads (DC-5) | ||
Ag/SnO2 WTOSF | Especially suitable for high inductive DC loads | ||
| Material/DODUCO-Designation | Properties |
|---|---|
| Ag/CdO DODURIT CdO |
High resistance against welding during current on switching for currents up to 5kA especially for powder metallurgical materials, Weld resistance increases with higher oxide contents, |
| Ag/SnO2 SISTADOX |
Environmentally friendly materials, Very high resistance against welding during current on switching, |
| Ag/ZnO DODURIT ZnO |
Environmentally friendly materials, High resistance against welding during current on switching |
Material | Application Examples |
|---|---|
Ag/CdO | Micro switches, Network relays, Wiring devices, Appliance switches, Main switches, contactors, Small (main) power switches |
Ag/SnO2 | Micro switches, Network relays, Automotive relays, Appliance switches, Main switches, contactors, Fault current protection relays (paired against Ag/C), (Main) Power switches |
Ag/ZnO | Wiring devices, AC relays, Appliance switches, Motor-protective circuit breakers (paired with Ag/Ni or Ag/C), Fault current circuit breakers paired againct Ag/C, (Main) Power switches |
Silver–Graphite (GRAPHOR)-Materials
Ag/C (GRAPHOR) contact materials are usually produced by powder metallurgy with graphite contents of 2 – 5 wt% Table 19 (Table 2.32). The earlier typical manufacturing process of single pressed tips by pressing - sintering - repressing (PSR) has been replaced in Europe for quite some time by extrusion. In North America and some other regions however the PSR process is still used to some extend mainly for cost reasons.
The extrusion of sintered billets is now the dominant manufacturing method for semi-finished AgC materials . The hot extrusion process results in a high density material with graphite particles stretched and oriented in the extrusion direction (Figs. 86 – 89)(Figs. 2.130 – 2.133). Depending on the extrusion method in either rod or strip form the graphite particles can be oriented in the finished contact tips perpendicular (GRAPHOR) or parallel (GRAPHOR D) to the switching contact surface Figure 89 (Fig. 2.131) and Figure 90 (Fig. 2.132).
Since the graphite particles in the Ag matrix of Ag/C materials prevent contact tips from directly being welded or brazed, a graphite free bottom layer is required. This is achieved by either burning out (de-graphitizing) the graphite selectively on one side of the tips or by compound extrusion of a Ag/C billet covered with a fine silver shell.
Ag/C contact materials exhibit on the one hand an extremely high resistance to contact welding but on the other have a low arc erosion resistance. This is caused by the reaction of graphite with the oxygen in the surrounding atmosphere at the high temperatures created by the arcing. The weld resistance is especially high for materials with the graphite particle orientation parallel to the arcing contact surface. Since the contact surface after arcing consists of pure silver the contact resistance stays consistently low during the electrical life of the contact parts.
A disadvantage of the Ag/C materials is their rather high erosion rate. In materials with parallel graphite orientation this can be improved if part of the graphite is incorporated into the material in the form of fibers (GRAPHOR DF), Figure 91 (Fig. 2.133). The weld resistance is determined by the total content of graphite particles.
Ag/C tips with vertical graphite particle orientation are produced in a specific sequence: Extrusion to rods, cutting of double thickness tips, burning out of graphite to a controlled layer thickness, and a second cutting to single tips. Such contact tips are especially well suited for applications which require both, a high weld resistance and a sufficiently high arc erosion resistance Table 20 (Table 2.33). For attachment of Ag/C tips welding and brazing techniques are applied.
welding the actual process depends on the material's graphite orientation. For Ag/C tips with vertical graphite orientation the contacts are assembled with single tips. For parallel orientation a more economical attachment starting with contact material in strip or profile tape form is used in integrated stamping and welding operations with the tape fed into the weld station, cut off to tip form and then welded to the carrier material before forming the final contact assembly part. For special low energy welding the Ag/C profile tapes GRAPHOR D and DF can be pre-coated with a thin layer of high temperature brazing alloys such as CuAgP.
In a rather limited way, Ag/C with 2 – 3 wt% graphite can be produced in wire form and headed into contact rivet shape with low head deformation ratios.
The main applications for Ag/C materials are protective switching devices such as miniature molded case circuit breakers, motor-protective circuit breakers, and fault current circuit breakers, where during short circuit failures highest resistance against welding is required Table 21 (Table 2.34). For higher currents the low arc erosion resistance of Ag/C is compensated by asymmetrical pairing with more erosion resistant materials such as Ag/Ni and Ag/W.
Figure 84 Fig. 2.126: Strain hardening of Ag/C 96/4 D by cold working
Figure 85 Fig. 2.127: Softening of Ag/C 96/4 D after annealing
Figure 86 Fig. 2.128: Strain hardening of Ag/C DF by cold working
Figure 87 Fig. 2.129: Softening of Ag/C DF after annealing
Figure 88 Fig. 2.130: Micro structure of Ag/C 97/3: a) perpendicular to extrusion direction b) parallel to extrusion direction, 1) Ag/C contact layer, 2) Ag backing layer
Figure 89 Fig. 2.131: Micro structure of Ag/C 95/5: a) perpendicular to extrusion direction b) parallel to extrusion direction, 1) Ag/C contact layer, 2) Ag backing layer
Figure 90 Fig. 2.132: Micro structure of Ag/C 96/4 D: a) perpendicular to extrusion direction b) parallel to extrusion direction, 1) Ag/C contact layer, 2) Ag backing layer
Figure 91 Fig. 2.133: Micro structure of Ag/C DF: a) perpendicular to extrusion direction b) parallel to extrusion direction, 1) Ag/C contact layer, 2) Ag/Ni 90/10 backing layer
| Material/ DODUCO- Designation |
Silver Content [wt%] |
Density [g/cm3] |
Melting Point [°C] |
Electrical Resistivity [μΩ·cm] |
Electrical Conductivity [% IACS] [MS/m] |
Vickers-Hardnes HV10 42 - 45 | |
|---|---|---|---|---|---|---|---|
| Ag/C 98/2 GRAPHOR 2 |
97.5 - 98.5 | 9.5 | 960 | 1.85 - 1.92 | 90 - 93 | 48 - 50 | 42 - 44 |
| Ag/C 97/3 GRAPHOR 3 |
96.5 - 97.5 | 9.1 | 960 | 1.92 - 2.0 | 86 - 90 | 45 - 48 | 41 - 43 |
| Ag/C 96/4 GRAPHOR 4 |
95.5 - 96.5 | 8.7 | 960 | 2.04 - 2.13 | 81 - 84 | 42 - 46 | 40 - 42 |
| Ag/C 95/5 GRAPHOR 5 |
94.5 - 95.5 | 8.5 | 960 | 2.12 - 2.22 | 78 - 81 | 40 - 44 | 40 - 60 |
| Ag/C 97/3D GRAPHOR 3D*) |
96.5 - 97.5 | 9.1 - 9.3 | 960 | 1.92 - 2.08 | 83 - 90 | 45 - 50 | 35 - 55 |
| Ag/C 96/4D GRAPHOR 4D*) |
95.5 - 96.5 | 8.8 - 9.0 | 960 | 2.04 - 2.22 | 78 - 84 | 43 - 47 | 35 - 60 |
| AgCDF GRAPHOR DF**) |
95.7 - 96.7 | 8.7 - 8.9 | 960 | 2.27 - 2.50 | 69 - 76 | 40 - 44 | |
*) Graphite particles parallel to switching surface
**) Graphite content 3.8 wt%, Graphite particles and fibers parallel to switching surface
Material/ DODUCO-Designation | Properties |
|---|---|
Ag/C GRAPHOR | Highest resistance against welding during make operations at high currents, High resistance against welding of closed contacts during short circuit, Increase of weld resistance with higher graphite contents, Low contact resistance, Low arc erosion resistance, especially during break operations, Higher arc erosion with increasing graphite contents, at the same time carbon build-up on switching chamber walls increases, GRAPHOR with vertical orientation has better arc erosion resistance, parallel orientation has better weld resistance, Limited arc moving properties, therefore paired with other materials, Limited formability, Can be welded and brazed with decarbonized backing, GRAPHOR DF is optimized for arc erosion resistance and weld resistance |
Material/ DODUCO Designation | Application Examples | Form of Supply |
|---|---|---|
Ag/C 98/2 GRAPHOR 2 | Motor circuit breakers, paired with Ag/Ni | Contact tips, brazed and welded contact parts, some contact rivets |
Ag/C 97/3 GRAPHOR 3 Ag/C 96/4 GRAPHOR 4 Ag/C 95/5 GRAPHOR 5 GRAPHOR 3D GRAPHOR 4D GRAPHOR DF | Circuit breakers, paired with Cu, Motor-protective circuit breakers, paired with Ag/Ni, Fault current circuit breakers, paired with Ag/Ni, Ag/W, Ag/WC, Ag/SnO2, Ag/ZnO, (Main) Power switches, paired with Ag/Ni, Ag/W | Contact tips, brazed and welded contact parts, some contact rivets with Ag/C97/3 |
Ag/C 97/3 GRAPHOR 3 Ag/C 96/4 GRAPHOR 4 Ag/C 95/5 GRAPHOR 5 GRAPHOR 3D GRAPHOR 4D GRAPHOR DF | Circuit breakers, paired with Cu, Motor-protective circuit breakers, paired with Ag/Ni, Fault current circuit breakers, paired with Ag/Ni, Ag/W, Ag/WC, Ag/SnO2, Ag/ZnO, (Main) Power switches, paired with Ag/Ni, Ag/W | Contact profiles (weld tapes), Contact tips, brazed and welded contact parts |