Difference between revisions of "Physikalische Eigenschaften der wichtigsten Metalle"
Doduco Admin (talk | contribs) |
Doduco Admin (talk | contribs) |
||
| (21 intermediate revisions by the same user not shown) | |||
| Line 246: | Line 246: | ||
!Ordnungszahl | !Ordnungszahl | ||
!Atommasse | !Atommasse | ||
| − | !Kristallstruktur [[#text- | + | !Kristallstruktur [[#text-reference|<sup>1</sup>]] |
| − | !Gitterparameter [[#text- | + | !Gitterparameter [[#text-reference|<sup>1</sup>]]<br />a oder b [[#text-reference|<sup>2</sup>]]<br />[10<sup>10</sup>m] |
| − | !Gitterparameter [[#text- | + | !Gitterparameter [[#text-reference|<sup>1</sup>]]<br />a oder b [[#text-reference|<sup>2</sup>]]<br />[10<sup>-10</sup>m] |
!Arbeitsleistung<br />[eV] | !Arbeitsleistung<br />[eV] | ||
!Ionisierungspotenzial<br/>[eV] | !Ionisierungspotenzial<br/>[eV] | ||
| Line 376: | Line 376: | ||
|6 | |6 | ||
|12,01 | |12,01 | ||
| − | |hcp-Schichtgitter[[#text- | + | |hcp-Schichtgitter[[#text-reference|<sup>3</sup>]] |
|2,456 | |2,456 | ||
|6,696 | |6,696 | ||
| Line 476: | Line 476: | ||
|80 | |80 | ||
|200,59 | |200,59 | ||
| − | |rhl[[#text- | + | |rhl[[#text-reference|<sup>4</sup>]] |
| − | |3,061[[#text- | + | |3,061[[#text-reference|<sup>4</sup>]] |
| | | | ||
|4,5 | |4,5 | ||
| Line 603: | Line 603: | ||
|- | |- | ||
|} | |} | ||
| − | <div id="text- | + | <div id="text-reference"><sub>1</sub> bei 20°C</div> |
| − | <div id="text- | + | <div id="text-reference"><sub>2</sub> Für rhomboedrische Kristalle wird der Rhomboederwinkel α in Winkelgraden und Minuten angegeben, für orthorhombische Kristalle wird der Parameter β in m x 10<sup>-10</sup> angegeben</div> |
| − | <div id="text- | + | <div id="text-reference"><sub>3</sub> α-Kristall</div> |
| − | <div id="text- | + | <div id="text-reference"><sub>4</sub> bei -50°C</div> |
</figtable> | </figtable> | ||
| − | fcc = kubisch-flächenzentriert // bcc = kubisch-körperzentriert // hcp = sechseckig dicht kugelförmig | + | fcc = kubisch-flächenzentriert // bcc = kubisch-körperzentriert // hcp = sechseckig dicht kugelförmig |
ort = orthorhombisch // tet = tetragonal // rhl = rhomboedrisch | ort = orthorhombisch // tet = tetragonal // rhl = rhomboedrisch | ||
<br/> | <br/> | ||
<br/> | <br/> | ||
| − | |||
<figtable id="tab:Thermische Eigenschaften der wichtigsten Metalle"> | <figtable id="tab:Thermische Eigenschaften der wichtigsten Metalle"> | ||
<caption>'''Thermische Eigenschaften der wichtigsten Metalle'''</caption> | <caption>'''Thermische Eigenschaften der wichtigsten Metalle'''</caption> | ||
| Line 619: | Line 618: | ||
|- | |- | ||
!Element/Metall | !Element/Metall | ||
| − | !Spezifische Wärme [[#text- | + | !Spezifische Wärme [[#text-reference|<sup>1</sup>]]<br/>[kJ/(K*kg)] |
!ErweichungsTemperatur<br/>[°C] | !ErweichungsTemperatur<br/>[°C] | ||
!Schmelzpunkt<br/>[°C] | !Schmelzpunkt<br/>[°C] | ||
| Line 642: | Line 641: | ||
| -6,5 | | -6,5 | ||
|- | |- | ||
| − | | | + | |Aluminium |
| − | | | + | |Al |
| + | |13 | ||
| + | |26,98 | ||
| + | |foc | ||
| + | |4,049 | ||
| | | | ||
| − | | | + | |4,08 - 4,3 |
| − | + | |5,98 | |
| − | + | |4,08 - 4,3 | |
| − | | | + | |5,98 |
| − | | | ||
| − | |||
| − | | | ||
| − | |||
|- | |- | ||
| − | | | + | |Aluminium |
| − | | | + | |Al |
| − | | | + | |13 |
| − | | | + | |26,98 |
| − | | | + | |foc |
| − | |4, | + | |4,049 |
| − | |||
| − | |||
| − | |||
| − | |||
| | | | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
|- | |- | ||
| − | | | + | |Aluminium |
| − | | | + | |Al |
| − | | | + | |13 |
| − | | | + | |26,98 |
| − | | | + | |foc |
| − | |4, | + | |4,049 |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| | | | ||
| − | | | + | |4,08 - 4,3 |
| − | + | |5,98 | |
| − | + | |4,08 - 4,3 | |
| − | | | + | |5,98 |
| − | |||
| − | | | ||
| − | |||
| − | | | ||
|- | |- | ||
| − | | | + | |Aluminium |
| − | | | + | |Al |
| − | + | |13 | |
| − | + | |26,98 | |
| − | + | |foc | |
| − | | | + | |4,049 |
| − | | | ||
| − | |||
| − | | | ||
| − | | | ||
| | | | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
|- | |- | ||
| − | | | + | |Aluminium |
| − | | | + | |Al |
| − | + | |13 | |
| − | + | |26,98 | |
| − | | | + | |foc |
| − | | | + | |4,049 |
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| | | | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
|- | |- | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|- | |- | ||
| − | | | + | |Aluminium |
| − | | | + | |Al |
| − | + | |13 | |
| − | + | |26,98 | |
| − | + | |foc | |
| − | + | |4,049 | |
| − | |||
| − | |||
| − | | | ||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | | | ||
| | | | ||
| − | | | + | |4,08 - 4,3 |
| − | | | + | |5,98 |
| − | | | + | |4,08 - 4,3 |
| − | + | |5,98 | |
| − | |||
| − | | | ||
| − | |||
| − | |||
|- | |- | ||
| − | | | + | |Aluminium |
| − | | | + | |Al |
| − | | | + | |13 |
| − | | | + | |26,98 |
| − | + | |foc | |
| − | + | |4,049 | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | | | ||
| − | | | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| | | | ||
| − | | | + | |4,08 - 4,3 |
| − | + | |5,98 | |
| − | + | |4,08 - 4,3 | |
| − | + | |5,98 | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |5, | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|- | |- | ||
|Aluminium | |Aluminium | ||
| − | | | + | |Al |
| − | + | |13 | |
| − | + | |26,98 | |
| − | + | |foc | |
| − | + | |4,049 | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | | | ||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | | | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| | | | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
| + | |4,08 - 4,3 | ||
| + | |5,98 | ||
|- | |- | ||
| − | | | + | |Indium |
| − | | | + | |In |
| − | + | |49 | |
| − | + | |114,82 | |
| − | + | |tet | |
| − | + | |4,594 | |
| − | + | |4,951 | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | | | ||
| − | | | ||
| − | |||
| − | | | ||
| − | |4, | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|4,0 | |4,0 | ||
| − | | | + | |5,79 |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|- | |- | ||
|Iridium | |Iridium | ||
| − | | | + | |Ir |
| − | | | + | |77 |
| − | | | + | |192,20 |
| − | | | + | |fcc |
| − | + | |3,839 | |
| − | |||
| − | |||
| − | |||
| − | | | ||
| | | | ||
| + | |4,6 - 5,3 | ||
| + | |9,1 | ||
|- | |- | ||
| − | | | + | |Kobalt |
| − | | | + | |Co |
| − | | | + | |27 |
| − | | | + | |58,93 |
| − | | | + | |hcp |
| − | | | + | |2,507 |
| − | | | + | |4,069 |
| − | | | + | |4,4 - 4,6 |
| − | + | |7,86 | |
| − | | | ||
| − | |||
|- | |- | ||
|Kohlenstoff (Graphit) | |Kohlenstoff (Graphit) | ||
| − | | | + | |C |
| − | | | + | |6 |
| − | | | + | |12,01 |
| − | | | + | |hcp-Schichtgitter[[#text-reference|<sup>3</sup>]] |
| − | | | + | |2,456 |
| − | | | + | |6,696 |
| − | | | + | |4,8 |
| − | | | + | |11,27 |
| − | | | ||
| − | |||
|- | |- | ||
|Kupfer | |Kupfer | ||
| − | | | + | |Cu |
| − | + | |29 | |
| − | | | + | |63,54 |
| − | | | + | |fcc |
| − | | | + | |3,615 |
| − | | | ||
| − | |||
| − | |||
| − | |||
| | | | ||
| + | |4,4 | ||
| + | |7,72 | ||
|- | |- | ||
|Magnesium | |Magnesium | ||
| − | | | + | |Mg |
| − | | | + | |12 |
| − | | | + | |24,31 |
| − | | | + | |hcp |
| − | | | + | |3,209 |
| − | | | + | |5,210 |
| − | | | + | |3,7 |
| − | + | |7,64 | |
| − | |||
| − | |||
|- | |- | ||
|Mangan | |Mangan | ||
| − | | | + | |Mn |
| − | | | + | |25 |
| − | | | + | |54,94 |
| + | |kubisch-komplex | ||
| + | |8,912 | ||
| | | | ||
| − | | | + | |3,8 - 4,1 |
| − | | | + | |6,0 |
| − | |||
| − | |||
| − | |||
| − | |||
|- | |- | ||
|Molybdän | |Molybdän | ||
| − | | | + | |Mo |
| − | | | + | |42 |
| − | | | + | |95,94 |
| − | | | + | |bcc |
| − | | | + | |3,147 |
| − | | | + | | |
| − | | | + | |4,1 - 4,5 |
| − | + | |7,18 | |
| − | | | ||
| − | |||
|- | |- | ||
|Nickel | |Nickel | ||
| − | | | + | |Ni |
| − | | | + | |28 |
| − | | | + | |58,71 |
| − | | | + | |fcc |
| − | | | + | |3,524 |
| − | |||
| − | |||
| − | |||
| − | |||
| | | | ||
| + | |5,0 - 5,2 | ||
| + | |7,63 | ||
|- | |- | ||
|Niob | |Niob | ||
| − | | | + | |Nb |
| − | | | + | |41 |
| − | | | + | |92,91 |
| − | | | + | |bcc |
| − | | | + | |3,301 |
| − | |||
| − | |||
| − | |||
| − | |||
| | | | ||
| + | |4,0 | ||
| + | |6,77 | ||
|- | |- | ||
|Osmium | |Osmium | ||
| − | | | + | |Os |
| − | | | + | |76 |
| − | | | + | |190,23 |
| − | | | + | |hcp |
| − | | | + | |2,734 |
| − | | | + | |4,320 |
| − | | | + | |4,5 |
| − | + | |8,7 | |
| − | | | ||
| − | |||
|- | |- | ||
|Palladium | |Palladium | ||
| − | | | + | |Pd |
| − | | | + | |46 |
| − | | | + | |106,40 |
| − | | | + | |fcc |
| − | |3, | + | |3,890 |
| | | | ||
| − | | | + | |4,5 - 5,0 |
| − | + | |8,34 | |
| − | | | ||
| − | |||
|- | |- | ||
|Platin | |Platin | ||
| − | | | + | |Pt |
| − | + | |78 | |
| − | | | + | |195,09 |
| − | | | + | |fcc |
| − | | | + | |3,931 |
| − | | | ||
| − | |||
| − | |||
| − | |||
| | | | ||
| + | |4,1 - 5,5 | ||
| + | |9,0 | ||
|- | |- | ||
|Quecksilber | |Quecksilber | ||
| − | | | + | |Hg |
| − | | | + | |80 |
| − | | | + | |200,59 |
| − | | | + | |rhl[[#text-reference|<sup>4</sup>]] |
| − | |4 | + | |3,061[[#text-reference|<sup>4</sup>]] |
| | | | ||
| − | | | + | |4,5 |
| − | | | + | |10,44 |
| − | |||
| − | |||
|- | |- | ||
|Rhenium | |Rhenium | ||
| − | | | + | |Re |
| − | | | + | |75 |
| − | | | + | |186,20 |
| − | | | + | |hcp |
| − | | | + | |2,760 |
| − | | | + | |4,458 |
| − | | | + | |4,7 - 5,0 |
| − | | | + | |7,8 |
| − | |||
| − | |||
|- | |- | ||
|Rhodium | |Rhodium | ||
| − | | | + | |Rh |
| − | + | |45 | |
| − | + | |102,91 | |
| − | | | + | |fcc |
| − | | | + | |3,804 |
| − | |||
| − | | | ||
| − | | | ||
| − | |||
| | | | ||
| + | |4,6 - 4,9 | ||
| + | |7,46 | ||
|- | |- | ||
|Ruthenium | |Ruthenium | ||
| − | | | + | |Ru |
| − | | | + | |44 |
| − | | | + | |101,07 |
| − | | | + | |hcp |
| − | | | + | |2,704 |
| − | | | + | |4,281 |
| − | | | + | |4,5 |
| − | + | |7,37 | |
| − | | | ||
| − | |||
|- | |- | ||
|Silber | |Silber | ||
| − | | | + | |Ag |
| − | | | + | |47 |
| + | |107,87 | ||
| + | |fcc | ||
| + | |4,086 | ||
| + | | | ||
|4,3 | |4,3 | ||
| − | | | + | |7,57 |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|- | |- | ||
|Tantal | |Tantal | ||
| − | | | + | |Ta |
| − | + | |73 | |
| − | | | + | |180,95 |
| − | | | + | |bcc |
| − | | | + | |3,303 |
| − | | | ||
| − | |||
| − | |||
| − | |||
| | | | ||
| + | |4,0 - 4,2 | ||
| + | |7,89 | ||
|- | |- | ||
|Titan | |Titan | ||
| − | | | + | |Ti |
| − | |2 | + | |2 |
| − | | | + | |47,90 |
| − | | | + | |hcp |
| − | | | + | |2,950 |
| − | + | |4,683 | |
| − | | | + | |4,0 - 4,4 |
| − | |0, | + | |6,83 |
| − | |||
| − | | | ||
|- | |- | ||
|Vanadium | |Vanadium | ||
| − | | | + | |V |
| − | | | + | |23 |
| − | | | + | |50,94 |
| − | | | + | |bcc |
| − | | | + | |3,039 |
| − | |||
| − | |||
| − | |||
| − | |||
| | | | ||
| + | |3,8 - 4,2 | ||
| + | |6,71 | ||
|- | |- | ||
|Bismut | |Bismut | ||
| − | | | + | |Bi |
| − | | | + | |83 |
| − | | | + | |208,98 |
| − | | | + | |rhl |
| − | | | + | |4,746 |
| − | |||
| − | |||
| − | |||
| − | |||
| | | | ||
| + | |4,1 - 4,5 | ||
| + | |8,0 | ||
|- | |- | ||
|Wolfram | |Wolfram | ||
| − | | | + | |W |
| − | + | |74 | |
| − | + | |183,85 | |
| − | + | |bcc | |
| − | | | + | |3,158 |
| − | | | ||
| − | | | ||
| − | | | ||
| | | | ||
| − | | | + | |4,3 - 5,0 |
| + | |7,98 | ||
|- | |- | ||
|Zink | |Zink | ||
| − | | | + | |Zn |
| − | | | + | |30 |
| − | | | + | |65,37 |
| − | | | + | |hcp |
| − | | | + | |2,665 |
| − | | | + | |4,947 |
| − | | | + | |3,1 - 4,3 |
| − | + | |9,39 | |
| − | | | ||
| − | |||
|- | |- | ||
|Zinn | |Zinn | ||
| − | | | + | |Sn |
| − | | | + | |50 |
| − | | | + | |118,69 |
| − | | | + | |tet |
| − | |3, | + | |5,831 |
| − | | | + | |3,181 |
| − | + | |3,6 - 4,1 | |
| − | | | + | |7,33 |
| − | |||
| − | |||
|- | |- | ||
|Zirconium | |Zirconium | ||
| − | | | + | |Zr |
| − | | | + | |40 |
| − | | | + | |91,22 |
| − | | | + | |hcp |
| − | | | + | |3,231 |
| − | | | + | |5,148 |
| − | | | + | |3,7 - 4,3 |
| − | + | |6,92 | |
| − | | | ||
| − | |||
|- | |- | ||
|} | |} | ||
| − | <div id="text- | + | <div id="text-reference"><sub>1</sub> bei 20°C</div> |
| − | < | + | |
| − | + | ||
| − | + | <figtable id="tab:Thermal Properties of the Most Important Metals"> | |
| − | + | [[File:Thermal-Properties-of-the-Most-Important-Metals.jpg|left|thumb|Tabelle 3: Thermische Eigenschaften der wichtigsten Metalle]] | |
</figtable> | </figtable> | ||
| − | + | <figtable id="tab:Electrical Properties of the Most Important Metals"> | |
| + | [[File:Electrical-Properties-of-the-Most-Important-Metals.jpg|left|thumb|Tabelle 4: Elektrische Eigenschaften der wichtigsten Metalle]] | ||
| + | </figtable> | ||
| + | </div> | ||
| + | <div class="clear"></div> | ||
===Referenzen=== | ===Referenzen=== | ||
Revision as of 10:02, 16 December 2022
In den nachfolgenden Tabellen sind die physikalischen Eigenschaften der gebräuchlichen
reinen Metalle sowie von Kohlenstoff aufgeführt (Tab. 1 - Tab. 4). Die
Werte können je nach Reinheitsgrad u. U. stark schwanken, teilweise sind sie
auch schwierig zu bestimmen und daher mit Unsicherheiten behaftet. Bei der
Zusammenstellung der Tabellen wurde versucht, aus den Angaben in der Literatur
diejenigen Werte auszuwählen, die als die wahrscheinlichsten anzusehen
sind. Einige Eigenschaften sind anisotrop, d.h. ihre Werte variieren je nach Kristallorientierung.
In solchen Fällen wurden - wenn möglich - die Werte für Vielkristalle
angegeben.
| Element/Metall | Dichte 1
[g/cm³] |
Elastizitätsmodul 1[GPa] | Schubmodul
[GPa] |
Querkontraktionszahl |
|---|---|---|---|---|
| Aluminium | 2.70 | 65 | 27 | 0.34 |
| Antimon | 6.62 | 56 | 20.4 | 0.28 |
| Beryllium | 1.85 | 298 | 150 | 0.12 |
| Blei | 11.36 | 14.5 | 6 | 0.44 |
| Cadmium | 8.65 | 57.5 | 29 | 0.30 |
| Chrom | 7.19 | 160 | 0.25 | |
| Eisen | 7.89 | 208 | 83 | 0.28 |
| Gallium | 5.91 | 9.6 | 0.46 | |
| Gold | 19.32 | 79 | 28 | 0.42 |
| Indium | 7.31 | 11 | 0.45 | |
| Iridium | 22.65 | 538 | 214 | 0.26 |
| Kobalt | 8.85 | 216 | 0.31 | |
| Kohlenstoff (Grafit) | 2.1-2.3 | 5 | ||
| Kupfer | 8.95 | 115 | 48 | 0.34 |
| Magnesium | 1.74 | 46 | 18 | 0.28 |
| Mangan | 7.43 | 165 | 77 | 0.24 |
| Molybdän | 10.21 | 347 | 122 | 0.30 |
| Nickel | 8.90 | 216 | 83 | 0.31 |
| Niob | 8.57 | 113 | 39 | 0.38 |
| Osmium | 22.61 | 570 | 220 | 0.25 |
| Palladium | 12.02 | 124 | 51 | 0.39 |
| Platin | 21.45 | 173 | 67 | 0.39 |
| Quecksilber | 13.55 | |||
| Rhenium | 21.04 | 480 | 215 | 0.26 |
| Rhodium | 12.41 | 386 | 153 | 0.26 |
| Ruthenium | 12.45 | 485 | 172 | 0.29 |
| Silber | 10.49 | 82 | 27 | 0.37 |
| Tantal | 16.60 | 188 | 70 | 0.35 |
| Titan | 4.51 | 120 | 43 | 0.34 |
| Vanadium | 6.10 | 136 | 52 | 0.36 |
| Wismut | 9.80 | 33 | 13 | 0.33 |
| Wolfram | 19.32 | 360 | 158 | 0.30 |
| Zink | 7.13 | 96 | 36 | 0.29 |
| Zinn | 7.30 | 47 | 18 | 0.33 |
| Zirkonium | 6.49 | 98 | 36 | 0.33 |
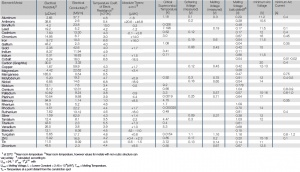
| Element/Metall | Chemisches Symbol |
Ordnungszahl | Atommasse | Kristallstruktur 1 | Gitterparameter 1 a oder b 2 [1010m] |
Gitterparameter 1 a oder b 2 [10-10m] |
Arbeitsleistung [eV] |
Ionisierungspotenzial [eV] |
|---|---|---|---|---|---|---|---|---|
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | |
| Antimon | Sb | 51 | 121,75 | rhl | 4,507 | 4,1 | 8,64 | |
| Beryllium | Be | 4 | 9,01 | hcp | 2,286 | 3,584 | 3,2 - 3,9 | 9,32 |
| Blei | Pb | 87 | 207,19 | fcc | 4,949 | 4,0 - 4,1 | 7,42 | |
| Cadmium | Cd | 48 | 112,40 | hcp | 2,979 | 5,617 | 3,7 - 4,1 | 8,99 |
| Chrom | Cr | 24 | 52,00 | bcc | 2,884 | 4,4 - 4,7 | 6,76 | |
| Eisen | Fe | 26 | 55,85 | bcc | 2,866 | 4,1 - 4,5 | 7,9 | |
| Gallium | Ga | 31 | 69,72 | ort | 4,524 | 7,661 | 3,8 - 4,1 | 6,0 |
| Gold | Au | 79 | 196,97 | fcc | 4,078 | 4,3 - 5,1 | 9,22 | |
| Indium | In | 49 | 114,82 | tet | 4,594 | 4,951 | 4,0 | 5,79 |
| Iridium | Ir | 77 | 192,20 | fcc | 3,839 | 4,6 - 5,3 | 9,1 | |
| Kobalt | Co | 27 | 58,93 | hcp | 2,507 | 4,069 | 4,4 - 4,6 | 7,86 |
| Kohlenstoff (Graphit) | C | 6 | 12,01 | hcp-Schichtgitter3 | 2,456 | 6,696 | 4,8 | 11,27 |
| Kupfer | Cu | 29 | 63,54 | fcc | 3,615 | 4,4 | 7,72 | |
| Magnesium | Mg | 12 | 24,31 | hcp | 3,209 | 5,210 | 3,7 | 7,64 |
| Mangan | Mn | 25 | 54,94 | kubisch-komplex | 8,912 | 3,8 - 4,1 | 6,0 | |
| Molybdän | Mo | 42 | 95,94 | bcc | 3,147 | 4,1 - 4,5 | 7,18 | |
| Nickel | Ni | 28 | 58,71 | fcc | 3,524 | 5,0 - 5,2 | 7,63 | |
| Niob | Nb | 41 | 92,91 | bcc | 3,301 | 4,0 | 6,77 | |
| Osmium | Os | 76 | 190,23 | hcp | 2,734 | 4,320 | 4,5 | 8,7 |
| Palladium | Pd | 46 | 106,40 | fcc | 3,890 | 4,5 - 5,0 | 8,34 | |
| Platin | Pt | 78 | 195,09 | fcc | 3,931 | 4,1 - 5,5 | 9,0 | |
| Quecksilber | Hg | 80 | 200,59 | rhl4 | 3,0614 | 4,5 | 10,44 | |
| Rhenium | Re | 75 | 186,20 | hcp | 2,760 | 4,458 | 4,7 - 5,0 | 7,8 |
| Rhodium | Rh | 45 | 102,91 | fcc | 3,804 | 4,6 - 4,9 | 7,46 | |
| Ruthenium | Ru | 44 | 101,07 | hcp | 2,704 | 4,281 | 4,5 | 7,37 |
| Silber | Ag | 47 | 107,87 | fcc | 4,086 | 4,3 | 7,57 | |
| Tantal | Ta | 73 | 180,95 | bcc | 3,303 | 4,0 - 4,2 | 7,89 | |
| Titan | Ti | 2 | 47,90 | hcp | 2,950 | 4,683 | 4,0 - 4,4 | 6,83 |
| Vanadium | V | 23 | 50,94 | bcc | 3,039 | 3,8 - 4,2 | 6,71 | |
| Bismut | Bi | 83 | 208,98 | rhl | 4,746 | 4,1 - 4,5 | 8,0 | |
| Wolfram | W | 74 | 183,85 | bcc | 3,158 | 4,3 - 5,0 | 7,98 | |
| Zink | Zn | 30 | 65,37 | hcp | 2,665 | 4,947 | 3,1 - 4,3 | 9,39 |
| Zinn | Sn | 50 | 118,69 | tet | 5,831 | 3,181 | 3,6 - 4,1 | 7,33 |
| Zirconium | Zr | 40 | 91,22 | hcp | 3,231 | 5,148 | 3,7 - 4,3 | 6,92 |
fcc = kubisch-flächenzentriert // bcc = kubisch-körperzentriert // hcp = sechseckig dicht kugelförmig
ort = orthorhombisch // tet = tetragonal // rhl = rhomboedrisch
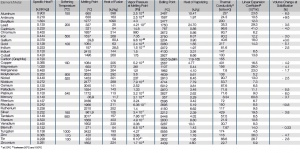
| Element/Metall | Spezifische Wärme 1 [kJ/(K*kg)] |
ErweichungsTemperatur [°C] |
Schmelzpunkt [°C] |
Fusionswärme [kJ/kg] |
Dampfdruck beim Schmelzpunkt [Pa] |
Siedepunkt [°C] |
Verdampfungstemperatur [kJ/g] |
Wärmeleitfähigkeit [W/(m*K)] |
Längenausdehnungskoeffizient [10-6m/K] |
Volumenänderung beim Erstarren [%] |
|---|---|---|---|---|---|---|---|---|---|---|
| Aluminium | 0,900 | 150 | 660 | 398 | 2,5x10-6 | 2467 | 10,47 | 237 | 23,6 | -6,5 |
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | 4,08 - 4,3 | 5,98 | |
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | 4,08 - 4,3 | 5,98 | |
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | 4,08 - 4,3 | 5,98 | |
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | 4,08 - 4,3 | 5,98 | |
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | 4,08 - 4,3 | 5,98 | |
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | 4,08 - 4,3 | 5,98 | |
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | 4,08 - 4,3 | 5,98 | |
| Aluminium | Al | 13 | 26,98 | foc | 4,049 | 4,08 - 4,3 | 5,98 | 4,08 - 4,3 | 5,98 | |
| Indium | In | 49 | 114,82 | tet | 4,594 | 4,951 | 4,0 | 5,79 | ||
| Iridium | Ir | 77 | 192,20 | fcc | 3,839 | 4,6 - 5,3 | 9,1 | |||
| Kobalt | Co | 27 | 58,93 | hcp | 2,507 | 4,069 | 4,4 - 4,6 | 7,86 | ||
| Kohlenstoff (Graphit) | C | 6 | 12,01 | hcp-Schichtgitter3 | 2,456 | 6,696 | 4,8 | 11,27 | ||
| Kupfer | Cu | 29 | 63,54 | fcc | 3,615 | 4,4 | 7,72 | |||
| Magnesium | Mg | 12 | 24,31 | hcp | 3,209 | 5,210 | 3,7 | 7,64 | ||
| Mangan | Mn | 25 | 54,94 | kubisch-komplex | 8,912 | 3,8 - 4,1 | 6,0 | |||
| Molybdän | Mo | 42 | 95,94 | bcc | 3,147 | 4,1 - 4,5 | 7,18 | |||
| Nickel | Ni | 28 | 58,71 | fcc | 3,524 | 5,0 - 5,2 | 7,63 | |||
| Niob | Nb | 41 | 92,91 | bcc | 3,301 | 4,0 | 6,77 | |||
| Osmium | Os | 76 | 190,23 | hcp | 2,734 | 4,320 | 4,5 | 8,7 | ||
| Palladium | Pd | 46 | 106,40 | fcc | 3,890 | 4,5 - 5,0 | 8,34 | |||
| Platin | Pt | 78 | 195,09 | fcc | 3,931 | 4,1 - 5,5 | 9,0 | |||
| Quecksilber | Hg | 80 | 200,59 | rhl4 | 3,0614 | 4,5 | 10,44 | |||
| Rhenium | Re | 75 | 186,20 | hcp | 2,760 | 4,458 | 4,7 - 5,0 | 7,8 | ||
| Rhodium | Rh | 45 | 102,91 | fcc | 3,804 | 4,6 - 4,9 | 7,46 | |||
| Ruthenium | Ru | 44 | 101,07 | hcp | 2,704 | 4,281 | 4,5 | 7,37 | ||
| Silber | Ag | 47 | 107,87 | fcc | 4,086 | 4,3 | 7,57 | |||
| Tantal | Ta | 73 | 180,95 | bcc | 3,303 | 4,0 - 4,2 | 7,89 | |||
| Titan | Ti | 2 | 47,90 | hcp | 2,950 | 4,683 | 4,0 - 4,4 | 6,83 | ||
| Vanadium | V | 23 | 50,94 | bcc | 3,039 | 3,8 - 4,2 | 6,71 | |||
| Bismut | Bi | 83 | 208,98 | rhl | 4,746 | 4,1 - 4,5 | 8,0 | |||
| Wolfram | W | 74 | 183,85 | bcc | 3,158 | 4,3 - 5,0 | 7,98 | |||
| Zink | Zn | 30 | 65,37 | hcp | 2,665 | 4,947 | 3,1 - 4,3 | 9,39 | ||
| Zinn | Sn | 50 | 118,69 | tet | 5,831 | 3,181 | 3,6 - 4,1 | 7,33 | ||
| Zirconium | Zr | 40 | 91,22 | hcp | 3,231 | 5,148 | 3,7 - 4,3 | 6,92 |
<figtable id="tab:Thermal Properties of the Most Important Metals">
Referenzen
Metals Handbook, Desk Edition: Chicago, IL, American Society of Metal, 1985
Landolt-Börnstein: Zahlenwerte und Funktionen. Springer-Verlag, Berlin-Göttingen-Heidelberg, 1959
Handbook of Chemistry and Physics, 70th Edition: CRC Press., Inc. Boca Raton, Florida, 1989 - 1990
Fluck, E.; Heumann, K., G.: Periodensystem der Elemente. Weinheim: VCH-Verlagsgesellschaft, 1986
Kieffer, R.; Jangg, G.; Ettmayer, P.: Sondermetalle. Springer- Verlag, Wien-New York, 1963
Hering, E.; Schulz, W.: Physik für Ingenieure (Periodensystem der Elemente). Düsseldorf: VDI-Verlag, 1988
Degussa AG (Hrsg.): Edelmetall-Taschenbuch. Hüthig-Verlag, Heidelberg, 1995
Slade, P.; G. (editor): Electrical Contacts Principles and Applications. Marcel Dekker, Inc., New York-Basel, 1999
Gerritsen, A.; N.: Metallic Conductivity in: Flügge, S.: Handbuch der Physik, Bd. 19, Springer-Verlag, Berlin-Göttingen-Heidelberg, 1956
Köster, W.; Franz, H.: Poisson,s Ratio for Metals and Alloys. Metallurg. Reviews 6 (1961)
Nesmeyanow, A., N.: Vapor Pressure of the Chemical Elements: Elsevier, Amsterdam-London-New York, 1963
Wyckoff, R., W., G.: Crystal Structures. Vol 1,New York, 1963