Difference between revisions of "Werkstoffe aus Platin-Metallen"
Doduco Admin (talk | contribs) |
Teitscheid (talk | contribs) (temp edit) |
||
| (11 intermediate revisions by 2 users not shown) | |||
| Line 61: | Line 61: | ||
teuren Gold. Zwischenzeitlich hatte der Palladiumpreis ein Niveau erreicht, das | teuren Gold. Zwischenzeitlich hatte der Palladiumpreis ein Niveau erreicht, das | ||
über dem des Goldes lag, so dass der Einsatz von Pd für Kontaktzwecke stark | über dem des Goldes lag, so dass der Einsatz von Pd für Kontaktzwecke stark | ||
| − | rückläufig war. Heute ( | + | rückläufig war. Heute (2011) liegt der Palladiumpreis bei ca. 50% des Goldpreises. |
Die Legierungen des Pt mit Ru, Ir, Ni und W wurden vor allem in elektromechanischen | Die Legierungen des Pt mit Ru, Ir, Ni und W wurden vor allem in elektromechanischen | ||
Bauelementen der Fernmeldetechnik und in hochwertigen Zündunterbrechern | Bauelementen der Fernmeldetechnik und in hochwertigen Zündunterbrechern | ||
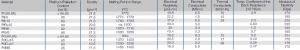
| − | verbreitet eingesetzt (<xr id="tab: | + | verbreitet eingesetzt (<xr id="tab:Physical Properties of platinum metals"/><!--(Tab. 2.7)-->). |
| − | <figtable id="tab: | + | <figtable id="tab:Physical Properties of platinum metals"> |
| − | + | [[File:Physical Properties of platinum metals.jpg|right|thumb|Physikalische Eigenschaften von Platin-Metallen und deren Legierungen]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
</figtable> | </figtable> | ||
| + | Today these components have been replaced in many applications by solid state technology and the usage of these materials is greatly reduced. Pd alloys however have a more significant importance. PdCu15 is widely used for example in automotive flasher relays. Because of their resistance to sulfide formation PdAg alloys are applied in various relay designs. The ability to thermally precipitation harden some multi component alloys based on PdAgAuPt they find special usage in wear resistant sliding contact applications. Pd44Ag38Cu15PtAuZn is a standard alloy in this group <xr id="tab:Mechanical_Properties_of_the_Platinum_Metals_and_their_Alloys"/><!--(Tab 2.8)--> und <xr id="tab:Contact_and_Switching_Properties_of_the_Platinum_Metals_and_their_Alloys"/><!--(Tab. 2.9)--> | ||
| − | + | Platinum and palladium alloys are mainly used similar to the gold based materials in the form of welded wire and profile segments but rarely as contact rivets. Because of the high precious metal prices joining technologies are used that allow the most economic application of the contact alloy in the area where functionally needed. Because of their resistance to material transfer they are used for DC applications and due to their higher arc erosion resistance they are applied for medium electrical loads up to about 30W in relays and switches <xr id="fig:Application_Examples_and_Form_of_Supply_for_Platinum_Metals_and_their_Alloys"/><!--(Table 2.10)-->. Multi-component alloys based on Pd with higher hardness and wear resistance are mainly used as spring arms in sliding contact systems and DC miniature motors. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<figtable id="tab:Mechanical_Properties_of_the_Platinum_Metals_and_their_Alloys"> | <figtable id="tab:Mechanical_Properties_of_the_Platinum_Metals_and_their_Alloys"> | ||
| − | <caption>''' | + | <caption>'''<!--Table 2.8:-->Mechanical Properties of the Platinum Metals and their Alloys'''</caption> |
<table class="twocolortable"> | <table class="twocolortable"> | ||
<tr> | <tr> | ||
| − | <th rowspan="2"> | + | <th rowspan="2">Material</th><th colspan="2">Tensile Strength [MPa]</th><th colspan="2">Elongation A [%]</th><th colspan="2">Vickers Hardness HV 1</th></tr> |
| − | <tr><th> | + | <tr><th>soft</th><th>70% cold worket</th><th>soft</th><th>70% cold worket</th><th>soft</th><th>70% cold worket</th></tr> |
<tr><td>Pt (99,95)</td><td>150</td><td>360</td><td>40</td><td>3</td><td>40</td><td>120</td></tr> | <tr><td>Pt (99,95)</td><td>150</td><td>360</td><td>40</td><td>3</td><td>40</td><td>120</td></tr> | ||
<tr><td>PtIr5</td><td>260</td><td>550</td><td>25</td><td>2</td><td>85</td><td>160</td></tr> | <tr><td>PtIr5</td><td>260</td><td>550</td><td>25</td><td>2</td><td>85</td><td>160</td></tr> | ||
| Line 229: | Line 97: | ||
</figtable> | </figtable> | ||
| − | * | + | *maximum hardness |
<figtable id="tab:Contact_and_Switching_Properties_of_the_Platinum_Metals_and_their_Alloys"> | <figtable id="tab:Contact_and_Switching_Properties_of_the_Platinum_Metals_and_their_Alloys"> | ||
<table class="twocolortable"> | <table class="twocolortable"> | ||
| − | <caption> '''<!--Table 2.9:--> | + | <caption> '''<!--Table 2.9:-->Contact and Switching Properties of the Platinum Metals and their Alloys'''</caption> |
| − | <tr><th><p class="s11"> | + | <tr><th><p class="s11">Material</p></th><th><p class="s12">Properties<th colspan="2"></p></th></tr><tr><td><p class="s11">Pt</p></td><td><p class="s12">Very high corrosion resistance</p></td><td/></tr><tr><td><p class="s11">PtIr5 - 10</p></td><td><p class="s12">Very high corrosion resistance, low contact resistance</p></td><td><p class="s12">High arc erosion resistance, high hardness</p></td></tr><tr><td><p class="s11">PtRu10</p></td><td><p class="s12">Very high corrosion resistance, low welding tendency</p></td><td><p class="s12">Low contact resistance, very</p><p class="s12">high hardness</p></td></tr><tr><td><p class="s11">PtNi8</p></td><td><p class="s12">Low material transfer tendency</p></td><td><p class="s12">Very high hardness</p></td></tr><tr><td><p class="s11">PtW5</p></td><td><p class="s12">Low material transfer tendency</p></td><td><p class="s12">High hardness</p></td></tr><tr><td><p class="s11">Pd</p></td><td><p class="s12">Strong tendency to “Brown Powder” formation</p></td><td><p class="s12">Less arc erosion resistant than Pt</p></td></tr><tr><td><p class="s11">PdCu15</p><p class="s11">PdCu40</p></td><td><p class="s12">Tendency to “Brown Powder” formation</p></td><td><p class="s12">Mostly resistant to material</p><p class="s12">transfer, high hardness</p></td></tr><tr><td><p class="s11">PdNi5</p></td><td><p class="s12">Strong tendency to “Brown Powder” formation</p></td><td><p class="s12">Low welding tendency</p></td></tr><tr><td><p class="s11">Pd44Ag38Cu15</p><p class="s11">PtAuZn</p></td><td><p class="s12">High mechanical wear resistance</p></td><td><p class="s12">Standard material for sliding</p><p class="s12">contact brushes</p></td></tr></table> |
</figtable> | </figtable> | ||
| Line 241: | Line 109: | ||
<figtable id="fig:Application_Examples_and_Form_of_Supply_for_Platinum_Metals_and_their_Alloys"> | <figtable id="fig:Application_Examples_and_Form_of_Supply_for_Platinum_Metals_and_their_Alloys"> | ||
<table class="twocolortable"> | <table class="twocolortable"> | ||
| − | <caption>'''<!--Table 2.10:--> | + | <caption>'''<!--Table 2.10:-->Application Examples and Form of Supply for Platinum Metals and their Alloys'''</caption> |
| − | <tr><th><p class="s11"> | + | <tr><th><p class="s11">Material</p></th><th><p class="s12">Application Examples</p></th><th><p class="s12">Forms of Supply</p></th></tr><tr><td><p class="s11">Pt (99,95)</p></td><td><p class="s12">Relays</p></td><td><p class="s12">Contact rivets, welded contact parts</p></td></tr><tr><td><p class="s11">PtIr5</p><p class="s11">PtIr10</p><p class="s11">PtRu10</p><p class="s11">PtNi8</p><p class="s11">PtW5</p></td><td><p class="s12">Relays, sliding contact systems,</p><p class="s12">automotive ignition breaker points</p></td><td><p class="s12">'''Semi-finished Contact Materials''':</p><p class="s12">Wire, seam-welded contact profiles</p><p class="s12">'''Contact Parts:'''</p><p class="s12">Tips, wire-formed parts, solid and composite contact rivets, welded contact parts</p></td></tr><tr><td><p class="s11">Pd (99,95)</p><p class="s11">PdNi5</p></td><td><p class="s12">Relays</p></td><td><p class="s12">Micro-profiles (weld tapes), contact rivets, welded contact parts</p></td></tr><tr><td><p class="s11">PdCu15</p><p class="s11">PdCu40</p></td><td><p class="s12">Automotive flasher relays</p></td><td><p class="s12">Micro-profiles, composite contact rivets</p></td></tr><tr><td><p class="s11">Pd35AuAgPt</p><p class="s11">Pd44Ag38Cu15</p><p class="s11">PtAuZn</p><p class="s11">Pd40Co40W20</p></td><td><p class="s12">Potentiometers, slip rings, miniature</p><p class="s12">DC motors</p></td><td><p class="s12">Wire-formed parts, welded wire segments, multi-arm sliding contact brushes</p></td></tr></table> |
</figtable> | </figtable> | ||
| + | |||
| + | |||
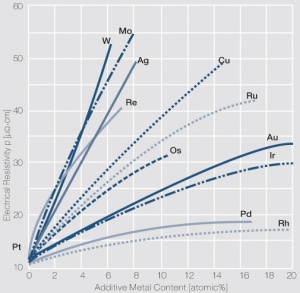
| + | <xr id="fig:Influence_of_1-20_atom%_of_different_additive_metals_on_the_electrical_resistivit_ p_of_platinum_(Degussa)"/>Influence of 1-20 atom% of different additive metals on the electrical resistivity p of platinum (Degussa) | ||
| + | |||
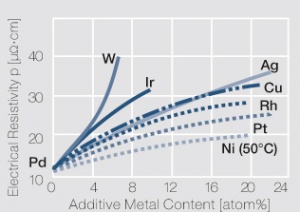
| + | <xr id="fig:Influence_of_1-20_atom%_of_different_additive_metals_on_the_electrical_resistivit_ p_of_platinum"/> | ||
| + | Influence of 1-22 atom% of different additive metals on the electrical resistivity p of palladium | ||
| + | |||
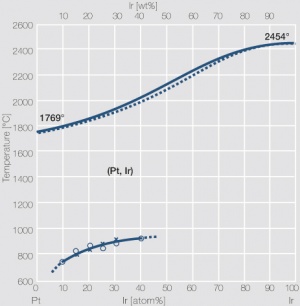
| + | <xr id="fig:Phase_diagram_of_platinum-iridium"/>Fig. 2.27: Phase diagram of platinum-iridium | ||
| + | |||
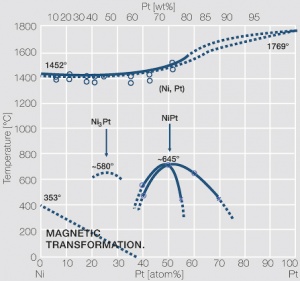
| + | <xr id="fig:Phase_diagram_of_platinum-nickel"/> | ||
| + | Fig. 2.28: Phase diagram of platinum-nickel | ||
| + | |||
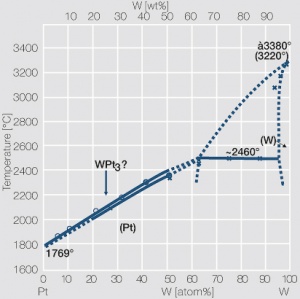
| + | <xr id="fig:Phase_diagram_of_platinum-tungsten"/> | ||
| + | Fig. 2.29: Phase diagram of platinum-tungsten | ||
| + | |||
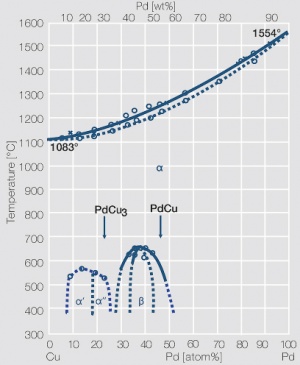
| + | <xr id="fig:Phase_diagram_of_platinum-copper"/> | ||
| + | Fig. 2.30: Phase diagram of palladium-copper | ||
| + | |||
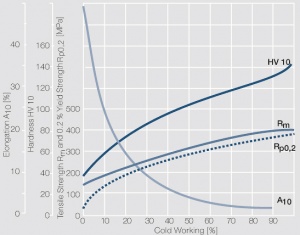
| + | <xr id="fig:Strain_hardening_of_Pt_by_cold_working"/> | ||
| + | Fig. 2.31: Strain hardening of Pt by cold working | ||
| + | |||
| + | <xr id="fig:Softening_of_Pt_after_annealing_for_0.5_hrs_after_80%_cold_working"/> | ||
| + | Fig. 2.32: Softening of Pt after annealing for 0.5 hrs after 80% cold working | ||
| + | |||
| + | <xr id="fig:Strain_hardening_of_PtIr5_by_cold_working"/> | ||
| + | Fig. 2.33: Strain hardening of PtIr5 by cold working | ||
| + | |||
| + | <xr id="fig:Softening_of_PtIr5_after_annealing_for_1_hr_after_different degrees_of_cold_working"/> | ||
| + | Fig. 2.34: Softening of PtIr5 after annealing for 1 hr after different degrees of cold working | ||
| + | |||
| + | <xr id="fig:Strain_hardening_of_PtNi8_by_cold_working"/>Fig. 2.35: Strain hardening of PtNi8 by cold working | ||
| + | |||
| + | <xr id="fig:Softening_of_PtNi8_after_annealing_for_1_hr_after_80%_cold_working"/> | ||
| + | Fig. 2.36: Softening of PtNi8 after annealing for 1 hr after 80% cold working | ||
| + | |||
| + | <xr id="fig:Strain_hardening_of_PtW5_by_cold_working"/>Fig. 2.37: Strain hardening of PtW5 by cold working | ||
| + | |||
| + | <xr id="fig:Softening_of_PtW5_after_annealing_for_1_hr_after_80%_cold_working"/>Fig. 2.38: Softening of PtW5 after annealing for 1hr after 80% cold working | ||
| + | |||
| + | <xr id="fig:Strain_hardening_of_Pd_99.99_by_cold_working"/>Fig. 2.39: Strain hardening of Pd 99.99 by cold working | ||
| + | |||
| + | <xr id="fig:Strain_hardening_of_PdCu15_by_cold_working"/>Fig. 2.40: Strain hardening of PdCu15 by cold working | ||
| + | |||
| + | <xr id="fig:Softening_of_PdCu15_after_annealing_for_0.5_hrs"/>Fig. 2.41: Softening of PdCu15 after annealing for 0.5 hrs | ||
| + | |||
| + | <xr id="fig:Strain_hardening_of_PdCu40_by_cold_working"/>Fig. 2.42: Strain hardening of PdCu40 by cold working | ||
| + | |||
| + | <xr id="fig:Softening_of_PdCu40_after_annealing_for_0.5_hrs_after_80%_cold_working"/>Fig. 2.43: Softening of PdCu40 after annealing for 0.5 hrs after 80% cold working | ||
| + | |||
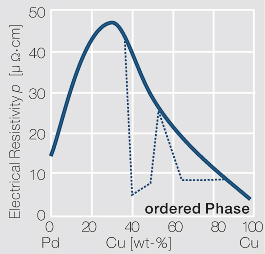
| + | <xr id="fig:Electrical_resistivity_p_of_PdCu_alloys"/>Fig. 2.44: Electrical resistivity p of PdCu alloys with and without an annealing step for forming an ordered phase | ||
| + | |||
| Line 250: | Line 169: | ||
<figure id="fig:Influence_of_1-20_atom%_of_different_additive_metals_on_the_electrical_resistivit_ p_of_platinum_(Degussa)"> | <figure id="fig:Influence_of_1-20_atom%_of_different_additive_metals_on_the_electrical_resistivit_ p_of_platinum_(Degussa)"> | ||
| − | [[File:Influence of platinum degussa.jpg|left|thumb|<caption> | + | [[File:Influence of platinum degussa.jpg|left|thumb|<caption>Influence of 1- 20 atom% of different additive metals on the electrical resistivity p of platinum (Degussa)</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Influence_of_1-20_atom%_of_different_additive_metals_on_the_electrical_resistivit_ p_of_platinum"> | <figure id="fig:Influence_of_1-20_atom%_of_different_additive_metals_on_the_electrical_resistivit_ p_of_platinum"> | ||
| − | [[File:Influence of palladium.jpg|left|thumb|<caption> | + | [[File:Influence of palladium.jpg|left|thumb|<caption>Influence of 1-22 atom% of different additive metals on the electrical resistivity p of palladium</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Phase_diagram_of_platinum-iridium"> | <figure id="fig:Phase_diagram_of_platinum-iridium"> | ||
| − | [[File:Phase diagram of platinum iridium.jpg|left|thumb|<caption> | + | [[File:Phase diagram of platinum iridium.jpg|left|thumb|<caption>Fig. 2.27:Phase diagram of platinum-iridium</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Phase_diagram_of_platinum-nickel"> | <figure id="fig:Phase_diagram_of_platinum-nickel"> | ||
| − | [[File:Phase diagram of platinum nickel.jpg|left|thumb|<caption> | + | [[File:Phase diagram of platinum nickel.jpg|left|thumb|<caption>Fig. 2.28:Phase diagram of platinum-nickel</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Phase_diagram_of_platinum-tungsten"> | <figure id="fig:Phase_diagram_of_platinum-tungsten"> | ||
| − | [[File:Phase diagram of palladium copper.jpg|left|thumb|<caption> | + | [[File:Phase diagram of palladium copper.jpg|left|thumb|<caption>Fig. 2.29:Phase diagram of platinum-tungsten</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Phase_diagram_of_platinum-copper"> | <figure id="fig:Phase_diagram_of_platinum-copper"> | ||
| − | [[File:Phase diagram of palladium copper2.jpg|left|thumb|<caption> | + | [[File:Phase diagram of palladium copper2.jpg|left|thumb|<caption>Fig. 2.30: Phase diagram of palladium-copper</caption>]] |
</figure> | </figure> | ||
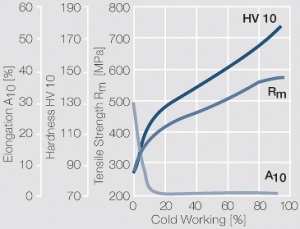
<figure id="fig:Strain_hardening_of_Pt_by_cold_working"> | <figure id="fig:Strain_hardening_of_Pt_by_cold_working"> | ||
| − | [[File:Strain hardening of Pt by cold working.jpg|left|thumb|<caption> | + | [[File:Strain hardening of Pt by cold working.jpg|left|thumb|<caption>Fig. 2.31: Strain hardening of Pt by cold working</caption>]] |
</figure> | </figure> | ||
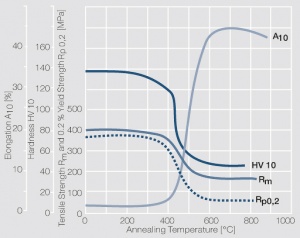
<figure id="fig:Softening_of_Pt_after_annealing_for_0.5_hrs_after_80%_cold_working"> | <figure id="fig:Softening_of_Pt_after_annealing_for_0.5_hrs_after_80%_cold_working"> | ||
| − | [[File:Softening of Pt after annealing.jpg|left|thumb|<caption> | + | [[File:Softening of Pt after annealing.jpg|left|thumb|<caption>Fig. 2.32: Softening of Pt after annealing for 0.5 hrs after 80% cold working</caption>]] |
</figure> | </figure> | ||
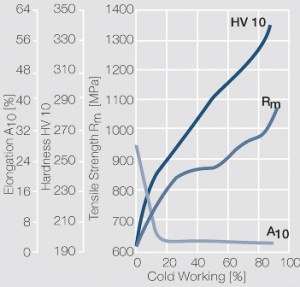
<figure id="fig:Strain_hardening_of_PtIr5_by_cold_working"> | <figure id="fig:Strain_hardening_of_PtIr5_by_cold_working"> | ||
| − | [[File:Strain hardening of PtIr5 by cold working.jpg|left|thumb|<caption> | + | [[File:Strain hardening of PtIr5 by cold working.jpg|left|thumb|<caption>Fig. 2.33: Strain hardening of PtIr5 by cold working</caption>]] |
</figure> | </figure> | ||
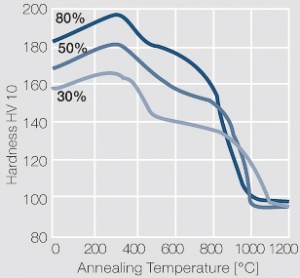
<figure id="fig:Softening_of_PtIr5_after_annealing_for_1_hr_after_different degrees_of_cold_working"> | <figure id="fig:Softening_of_PtIr5_after_annealing_for_1_hr_after_different degrees_of_cold_working"> | ||
| − | [[File:Softening of PtIr5 after annealing.jpg|left|thumb|<caption> | + | [[File:Softening of PtIr5 after annealing.jpg|left|thumb|<caption>Fig. 2.34: Softening of PtIr5 after annealing for 1 hr after different degrees of cold working</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Strain_hardening_of_PtNi8_by_cold_working"> | <figure id="fig:Strain_hardening_of_PtNi8_by_cold_working"> | ||
| − | [[File:Strain hardening of PtNi8 by cold working.jpg|left|thumb|<caption> | + | [[File:Strain hardening of PtNi8 by cold working.jpg|left|thumb|<caption>Fig. 2.35: Strain hardening of PtNi8 by cold working</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Softening_of_PtNi8_after_annealing_for_1_hr_after_80%_cold_working"> | <figure id="fig:Softening_of_PtNi8_after_annealing_for_1_hr_after_80%_cold_working"> | ||
| − | [[File:Softening of PtNi8 after annealing.jpg|left|thumb|<caption> | + | [[File:Softening of PtNi8 after annealing.jpg|left|thumb|<caption>Fig. 2.36: Softening of PtNi8 after annealing for 1 hr after 80% cold working</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Strain_hardening_of_PtW5_by_cold_working"> | <figure id="fig:Strain_hardening_of_PtW5_by_cold_working"> | ||
| − | [[File:Strain hardening of PtW5 by cold working.jpg|left|thumb|<caption> | + | [[File:Strain hardening of PtW5 by cold working.jpg|left|thumb|<caption>Fig. 2.37: Strain hardening of PtW5 by cold working</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Softening_of_PtW5_after_annealing_for_1_hr_after_80%_cold_working"> | <figure id="fig:Softening_of_PtW5_after_annealing_for_1_hr_after_80%_cold_working"> | ||
| − | [[File:Softening of PtW5 after annealing.jpg|left|thumb|<caption> | + | [[File:Softening of PtW5 after annealing.jpg|left|thumb|<caption>Fig. 2.38: Softening of PtW5 after annealing for 1 hr after 80% cold working</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Strain_hardening_of_Pd_99.99_by_cold_working"> | <figure id="fig:Strain_hardening_of_Pd_99.99_by_cold_working"> | ||
| − | [[File:Strain hardening of Pd-99 99by cold working.jpg|left|thumb|<caption> | + | [[File:Strain hardening of Pd-99 99by cold working.jpg|left|thumb|<caption>Fig. 2.39: Strain hardening of Pd 99.99 by cold working</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Strain_hardening_of_PdCu15_by_cold_working"> | <figure id="fig:Strain_hardening_of_PdCu15_by_cold_working"> | ||
| − | [[File:Strain hardening of PdCu15 by cold working.jpg|left|thumb|<caption> | + | [[File:Strain hardening of PdCu15 by cold working.jpg|left|thumb|<caption>Fig. 2.40: Strain hardening of PdCu15 by cold working</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Softening_of_PdCu15_after_annealing_for_0.5_hrs"> | <figure id="fig:Softening_of_PdCu15_after_annealing_for_0.5_hrs"> | ||
| − | [[File:Softening of PdCu15 after annealing.jpg|left|thumb|<caption> | + | [[File:Softening of PdCu15 after annealing.jpg|left|thumb|<caption>Softening of PdCu15 after annealing for 0.5 hrs</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Strain_hardening_of_PdCu40_by_cold_working"> | <figure id="fig:Strain_hardening_of_PdCu40_by_cold_working"> | ||
| − | [[File:Strain hardening of PdCu40 by cold working.jpg|left|thumb|<caption> | + | [[File:Strain hardening of PdCu40 by cold working.jpg|left|thumb|<caption>Strain hardening of PdCu40 by cold working</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Softening_of_PdCu40_after_annealing_for_0.5_hrs_after_80%_cold_working"> | <figure id="fig:Softening_of_PdCu40_after_annealing_for_0.5_hrs_after_80%_cold_working"> | ||
| − | [[File:Softening of PdCu40 after annealing.jpg|left|thumb|<caption> | + | [[File:Softening of PdCu40 after annealing.jpg|left|thumb|<caption>Softening of PdCu40 after annealing for 0.5 hrs after 80% cold working</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Electrical_resistivity_p_of_PdCu_alloys"> | <figure id="fig:Electrical_resistivity_p_of_PdCu_alloys"> | ||
| − | [[File:Electrical resistivity p of PdCu alloys.jpg|left|thumb|<caption> | + | [[File:Electrical resistivity p of PdCu alloys.jpg|left|thumb|<caption>Electrical resistivity p of PdCu alloys with and without an annealing step for forming an ordered phase</caption>]] |
</figure> | </figure> | ||
Revision as of 17:13, 22 September 2014
Zur Platingruppe zählen die Elemente Pt, Pd, Rh, Ru, Ir und Os (Table 1). Für Anwendungen in der Kontakttechnik haben Platin und Palladium als Legierungsgrundmetalle sowie Ruthenium und Iridium als Legierungsbestandteile praktische Bedeutung. Pt und Pd sind zwar ähnlich korrosionsbeständig wie Au, neigen aber aufgrund ihrer katalytischen Eigenschaften dazu, an der Kontaktoberfläche adsorbierte organische Dämpfe zu polymerisieren. Bei Reibbeanspruchung der Kontaktpartner entsteht dabei als Polymerisationsprodukt das sog. brown powder, das zu einer starken Erhöhung des Kontaktwiderstandes führen kann. Daher werden Pt und Pd nicht rein, sondern ausschließlich in Legierungsform für Kontaktzwecke eingesetzt.
| Elemente | Eigenschaften | Verarbeitung | Anwendungsformen |
|---|---|---|---|
| Ru Ruthenium |
Mattgrau bis silberweiß, sehr hart und spröde, gegen Säuren bei Anwesenheit von Sauerstoff beständig, oxidiert bei Erhitzen an Luft |
Aufdampfen, Sputtern, pulvermetallurgisch, Warmverformen nur bei 1200-1500°C möglich |
Pulver, in Blechen und Beschichtungen, in Drähten meist nur als Legierungsbestandteil |
| Rh Rhodium |
Nahezu silberweiß, sehr hart und spröde, in Säuren unlöslich, oxidiert an Luft bei Rotglut |
Galvanisch, Aufdampfen, Sputtern, nach Warmverformung bei 800-1000°C, Kaltverformen möglich |
Beschichtungen (galvanische Überzüge), Legierungsbestandteil, in geringem Umfang als Bleche und Drähte |
| Pd Palladium |
Mattweiß, duktil, gegen die meisten Säuren beständig, oxidiert bei Rotglut | Galvanisch, Aufdampfen, Sputtern, Kaltverformen |
Bleche, Bänder, Rohre, Drähte, Niete und Beschichtungen |
| Os Osmium |
Bläulichweiß, härtestes Platinmetall, sehr spröde, gegen nichtoxidierende Säuren beständig, an Luft leicht oxidierbar |
Pulvermetallurgisch | Pulver, Legierungsbestandteil |
| Ir Iridium |
Nahezu silberweiß, sehr hart und spröde, säurebeständig, oxidiert bei Rotglut |
Aufdampfen, Sputtern, pulvermetallurgisch, bei 1200-1500°C Warmverformen möglich |
Pulver, Legierungsbestandteil, in geringem, Umfang als Blech |
| Pt Platin |
Grauweiß, duktil, säurebeständig außer gegen Königswasser, HBr und HJ, oxidationsbeständig bei Rotglut |
Galvanisch, Aufdampfen, Sputtern, Kaltverformen |
Bleche, Bänder, Rohre, Drähte, Niete und Beschichtungen |
Rhodium kommt als massiver Kontaktwerkstoff nicht zum Einsatz, wird jedoch
als galvanisch aufgebrachte Schicht z.B. in Gleitkontaktsystemen verwendet.
Ruthenium dient hauptsächlich als Legierungskomponente in PdRu15. Die
Metalle Osmium und Iridium finden keine praktische Anwendung in der
Kontakttechnik.
Da Pd lange Zeit sehr preisstabil war, galt es als geeignete Alternative zu dem teuren Gold. Zwischenzeitlich hatte der Palladiumpreis ein Niveau erreicht, das über dem des Goldes lag, so dass der Einsatz von Pd für Kontaktzwecke stark rückläufig war. Heute (2011) liegt der Palladiumpreis bei ca. 50% des Goldpreises.
Die Legierungen des Pt mit Ru, Ir, Ni und W wurden vor allem in elektromechanischen Bauelementen der Fernmeldetechnik und in hochwertigen Zündunterbrechern verbreitet eingesetzt (Table 2).
Today these components have been replaced in many applications by solid state technology and the usage of these materials is greatly reduced. Pd alloys however have a more significant importance. PdCu15 is widely used for example in automotive flasher relays. Because of their resistance to sulfide formation PdAg alloys are applied in various relay designs. The ability to thermally precipitation harden some multi component alloys based on PdAgAuPt they find special usage in wear resistant sliding contact applications. Pd44Ag38Cu15PtAuZn is a standard alloy in this group Table 3 und Table 4
Platinum and palladium alloys are mainly used similar to the gold based materials in the form of welded wire and profile segments but rarely as contact rivets. Because of the high precious metal prices joining technologies are used that allow the most economic application of the contact alloy in the area where functionally needed. Because of their resistance to material transfer they are used for DC applications and due to their higher arc erosion resistance they are applied for medium electrical loads up to about 30W in relays and switches Table 5. Multi-component alloys based on Pd with higher hardness and wear resistance are mainly used as spring arms in sliding contact systems and DC miniature motors.
| Material | Tensile Strength [MPa] | Elongation A [%] | Vickers Hardness HV 1 | |||
|---|---|---|---|---|---|---|
| soft | 70% cold worket | soft | 70% cold worket | soft | 70% cold worket | |
| Pt (99,95) | 150 | 360 | 40 | 3 | 40 | 120 |
| PtIr5 | 260 | 550 | 25 | 2 | 85 | 160 |
| PtIr10 | 340 | 570 | 24 | 2 | 105 | 210 |
| PtRu10 | 650 | 1000 | 24 | 2 | 195 | 320 |
| PtNi8 | 640 | 950 | 22 | 2 | 200 | 320 |
| PtW5 | 530 | 860 | 21 | 2 | 150 | 270 |
| Pd (99,95) | 200 | 420 | 42 | 2 | 40 | 90 |
| PdCu15 | 400 | 780 | 38 | 2 | 90 | 220 |
| PdCu40 | 550 | 950 | 35 | 2 | 120 | 260 |
| PdNi5 | 340 | 700 | 25 | 2 | 95 | 200 |
| Pd35AuAgPt | 420* | |||||
| Pd44Ag38Cu15 PtAuZn | 405* | |||||
| Pd40Co40W20 | 680* | |||||
- maximum hardness
Table 4: Contact and Switching Properties of the Platinum Metals and their Alloys
Material | Properties | ||
|---|---|---|---|
Pt | Very high corrosion resistance | ||
PtIr5 - 10 | Very high corrosion resistance, low contact resistance | High arc erosion resistance, high hardness | |
PtRu10 | Very high corrosion resistance, low welding tendency | Low contact resistance, very high hardness | |
PtNi8 | Low material transfer tendency | Very high hardness | |
PtW5 | Low material transfer tendency | High hardness | |
Pd | Strong tendency to “Brown Powder” formation | Less arc erosion resistant than Pt | |
PdCu15 PdCu40 | Tendency to “Brown Powder” formation | Mostly resistant to material transfer, high hardness | |
PdNi5 | Strong tendency to “Brown Powder” formation | Low welding tendency | |
Pd44Ag38Cu15 PtAuZn | High mechanical wear resistance | Standard material for sliding contact brushes | |
Table 5: Application Examples and Form of Supply for Platinum Metals and their Alloys
Material | Application Examples | Forms of Supply |
|---|---|---|
Pt (99,95) | Relays | Contact rivets, welded contact parts |
PtIr5 PtIr10 PtRu10 PtNi8 PtW5 | Relays, sliding contact systems, automotive ignition breaker points | Semi-finished Contact Materials: Wire, seam-welded contact profiles Contact Parts: Tips, wire-formed parts, solid and composite contact rivets, welded contact parts |
Pd (99,95) PdNi5 | Relays | Micro-profiles (weld tapes), contact rivets, welded contact parts |
PdCu15 PdCu40 | Automotive flasher relays | Micro-profiles, composite contact rivets |
Pd35AuAgPt Pd44Ag38Cu15 PtAuZn Pd40Co40W20 | Potentiometers, slip rings, miniature DC motors | Wire-formed parts, welded wire segments, multi-arm sliding contact brushes |
Figure 1Influence of 1-20 atom% of different additive metals on the electrical resistivity p of platinum (Degussa)
Figure 2 Influence of 1-22 atom% of different additive metals on the electrical resistivity p of palladium
Figure 3Fig. 2.27: Phase diagram of platinum-iridium
Figure 4 Fig. 2.28: Phase diagram of platinum-nickel
Figure 5 Fig. 2.29: Phase diagram of platinum-tungsten
Figure 6 Fig. 2.30: Phase diagram of palladium-copper
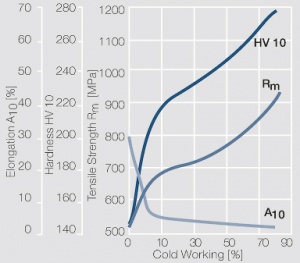
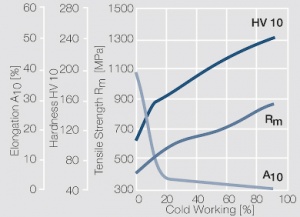
Figure 7 Fig. 2.31: Strain hardening of Pt by cold working
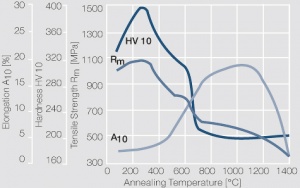
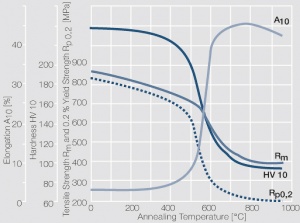
Figure 8 Fig. 2.32: Softening of Pt after annealing for 0.5 hrs after 80% cold working
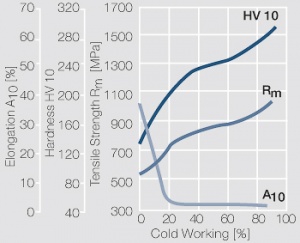
Figure 9 Fig. 2.33: Strain hardening of PtIr5 by cold working
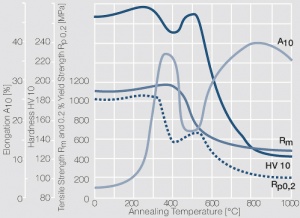
Figure 10 Fig. 2.34: Softening of PtIr5 after annealing for 1 hr after different degrees of cold working
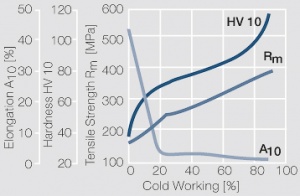
Figure 11Fig. 2.35: Strain hardening of PtNi8 by cold working
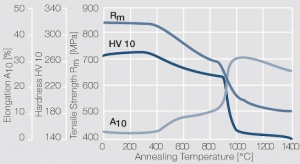
Figure 12 Fig. 2.36: Softening of PtNi8 after annealing for 1 hr after 80% cold working
Figure 13Fig. 2.37: Strain hardening of PtW5 by cold working
Figure 14Fig. 2.38: Softening of PtW5 after annealing for 1hr after 80% cold working
Figure 15Fig. 2.39: Strain hardening of Pd 99.99 by cold working
Figure 16Fig. 2.40: Strain hardening of PdCu15 by cold working
Figure 17Fig. 2.41: Softening of PdCu15 after annealing for 0.5 hrs
Figure 18Fig. 2.42: Strain hardening of PdCu40 by cold working
Figure 19Fig. 2.43: Softening of PdCu40 after annealing for 0.5 hrs after 80% cold working
Figure 20Fig. 2.44: Electrical resistivity p of PdCu alloys with and without an annealing step for forming an ordered phase