Difference between revisions of "Aushärtbare Kupfer-Legierungen"
Doduco Admin (talk | contribs) (→Kupfer-Chrom-Legierungen) |
Teitscheid (talk | contribs) (temp edit) |
||
| (17 intermediate revisions by 2 users not shown) | |||
| Line 10: | Line 10: | ||
Temperatur rasch abnehmende Löslichkeit des Berylliums im Kupfer. Wie aus | Temperatur rasch abnehmende Löslichkeit des Berylliums im Kupfer. Wie aus | ||
dem Zustandsschaubild für CuBe ersichtlich, sind bei ca. 780°C 2,4 Massen-% | dem Zustandsschaubild für CuBe ersichtlich, sind bei ca. 780°C 2,4 Massen-% | ||
| − | Be in Kupfer löslich | + | Be in Kupfer löslich <xr id="fig:Phase_diagram_of_copperberyllium_with_temperature_ranges_for_brazing_and_annealing_treatments"/><!--(Fig. 5.28)-->. In diesem Temperaturbereich wärmebehandelte |
CuBe-Legierungen sind homogen („lösungsglühen“). Der homogene Zustand | CuBe-Legierungen sind homogen („lösungsglühen“). Der homogene Zustand | ||
kann durch schnelles Abkühlen auf Raumtemperatur eingefroren werden („abschrecken“). | kann durch schnelles Abkühlen auf Raumtemperatur eingefroren werden („abschrecken“). | ||
| Line 16: | Line 16: | ||
von 325°C wird die gewünschte Ausscheidungshärtung erreicht, die einen | von 325°C wird die gewünschte Ausscheidungshärtung erreicht, die einen | ||
deutlichen Anstieg der Festigkeit und der elektrischen Leitfähigkeit von CuBe | deutlichen Anstieg der Festigkeit und der elektrischen Leitfähigkeit von CuBe | ||
| − | bewirkt | + | bewirkt <xr id="tab:Physical_Properties_of_Selected_Copper_Beryllium_Alloys"/><!--(Tab. 5.17)-->. Die erreichbaren Festigkeits- und Härtewerte sind abhängig |
| − | von der Glühtemperatur und Glühdauer sowie vom Umformgrad | + | von der Glühtemperatur und Glühdauer sowie vom Umformgrad (Tab. 5.18) und |
| − | + | <xr id="tab:Mechanical Properties of Selected Copper-Beryllium Alloys"/><!--(Table 5.18)--> and [[#figures7|(Figs. 43 – 75)]]<!--(Figs. 5.29 - 5.31)-->. | |
| Line 39: | Line 39: | ||
Kupfer-Legierungen ohne toxische bzw. deklarationspfichtige | Kupfer-Legierungen ohne toxische bzw. deklarationspfichtige | ||
Elemente z.B. CuNiCoSi zielt in Richtung CuBe-Ersatz. | Elemente z.B. CuNiCoSi zielt in Richtung CuBe-Ersatz. | ||
| + | |||
| + | <div id="figures7"> | ||
| + | |||
| + | <xr id="fig:Phase_diagram_of_copperberyllium_with_temperature_ranges_for_brazing_and_annealing_treatments"/><!--Fig. 5.28:--> Zustandsdiagramm Kupfer-Beryllium mit Temperaturbereichen für Hartlötungen und Wärmebehandlungen | ||
| + | |||
| + | <xr id="fig:Precipitation_hardening_of_CuBe2_at_325°C_after_different_cold_working"/><!--Fig. 5.29:--> Aushärtung von CuBe2 bei 325°C nach unterschiedlicher Kaltumformung | ||
| + | |||
| + | <xr id="fig:Precipitation_hardening_of_CuBe2_(soft)_at_325°C"/><!--Fig. 5.30:--> Precipitation hardening of CuBe2 (soft) at 325°C | ||
| + | |||
| + | <xr id="fig:Precipitation_hardening_of_CuBe2_(half hard)_at_different_annealing_temperatures"/><!--Fig. 5.31:--> Precipitation hardening of CuBe2 (half hard) at different annealing temperatures | ||
| + | </div> | ||
<div class="multiple-images"> | <div class="multiple-images"> | ||
| Line 51: | Line 62: | ||
<figure id="fig:Precipitation_hardening_of_CuBe2_(soft)_at_325°C"> | <figure id="fig:Precipitation_hardening_of_CuBe2_(soft)_at_325°C"> | ||
| − | [[File:Precipitation hardening of CuBe2 (soft) at 325C.jpg|left|thumb|<caption> | + | [[File:Precipitation hardening of CuBe2 (soft) at 325C.jpg|left|thumb|<caption>Precipitation hardening of CuBe2 (soft) at 325°C</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Precipitation_hardening_of_CuBe2_(half hard)_at_different_annealing_temperatures"> | <figure id="fig:Precipitation_hardening_of_CuBe2_(half hard)_at_different_annealing_temperatures"> | ||
| − | [[File:Precipitation hardening of CuBe2 half hard.jpg|left|thumb|<caption> | + | [[File:Precipitation hardening of CuBe2 half hard.jpg|left|thumb|<caption>Precipitation hardening of CuBe2 (half hard) at different annealing temperatures</caption>]] |
</figure> | </figure> | ||
</div> | </div> | ||
| Line 62: | Line 73: | ||
<figtable id="tab:Physical_Properties_of_Selected_Copper_Beryllium_Alloys"> | <figtable id="tab:Physical_Properties_of_Selected_Copper_Beryllium_Alloys"> | ||
| − | <caption>'''<!--Table 5.17:--> | + | <caption>'''<!--Table 5.17:-->Physical Properties of Selected Copper-Beryllium Alloys'''</caption> |
{| class="twocolortable" style="text-align: left; font-size: 12px" | {| class="twocolortable" style="text-align: left; font-size: 12px" | ||
|- | |- | ||
| − | ! | + | !Material<br />Designation<br />EN UNS |
| − | ! | + | !Composition<br />[wt%] |
| − | ! | + | !Density<br />[g/cm<sup>3</sup>] |
| − | !colspan="2" style="text-align:center"| | + | !colspan="2" style="text-align:center"|Electrical<br />Conductivity |
| − | ! | + | !Electrical<br />Resistivity<br />[μΩ·cm] |
| − | ! | + | !Thermal<br />Conductivity<br />[W/(m·K)] |
| − | ! | + | !Coeff. of Linear<br />Thermal<br />Expansion<br />[10<sup>-6</sup>/K] |
| − | ! | + | !Modulus of<br />Elasticity<br />[GPa] |
| − | ! | + | !Softening Temperature<br />(approx. 10% loss in<br />strength)<br />[°C] |
| − | ! | + | !Melting<br />Temp Range<br />[°C] |
|- | |- | ||
! | ! | ||
| Line 92: | Line 103: | ||
|Be 1.6 - 1.8<br />Co 0.3<br />Ni 0.3<br />Cu Rest | |Be 1.6 - 1.8<br />Co 0.3<br />Ni 0.3<br />Cu Rest | ||
|8.4 | |8.4 | ||
| − | |8 - 9 | + | |8 - 9<sup>a</sup><br />12 - 13<sup>b</sup><br />11<sup>c</sup> |
|14 - 16<br />21 - 22<br />19 | |14 - 16<br />21 - 22<br />19 | ||
| − | |11 - 12.5 | + | |11 - 12.5<sup>a</sup><br />7.7 - 8.3<sup>b</sup><br />9.1<sup>c</sup> |
|110 | |110 | ||
|17 | |17 | ||
| − | |125 | + | |125<sup>a</sup><br />135<sup>b</sup> |
|ca. 380 | |ca. 380 | ||
|890 - 1000 | |890 - 1000 | ||
| Line 104: | Line 115: | ||
|Be 1.8 - 2.1<br />Co 0.3<br />Ni 0.3<br />Cu Rest | |Be 1.8 - 2.1<br />Co 0.3<br />Ni 0.3<br />Cu Rest | ||
|8.3 | |8.3 | ||
| − | |8 - 9 | + | |8 - 9<sup>a</sup><br />12 - 13<sup>b</sup><br />11<sup>c</sup> |
|14 - 16<br />21 - 22<br />19 | |14 - 16<br />21 - 22<br />19 | ||
| − | |11 - 12.5 | + | |11 - 12.5<sup>a</sup><br />7.7 - 8.3<sup>b</sup><br />9.1<sup>c</sup> |
|110 | |110 | ||
|17 | |17 | ||
| − | |125 | + | |125<sup>a</sup><br />135<sup>b</sup> |
|ca. 380 | |ca. 380 | ||
|870 - 980 | |870 - 980 | ||
| Line 116: | Line 127: | ||
|Co 2.0 - 2.8<br />Be 0.4 - 0.7<br />Ni 0.3<br />Cu Rest | |Co 2.0 - 2.8<br />Be 0.4 - 0.7<br />Ni 0.3<br />Cu Rest | ||
|8.8 | |8.8 | ||
| − | |11 - 14 | + | |11 - 14<sup>a</sup><br />25 - 27<sup>b</sup><br />27 - 34<sup>c</sup> |
|19 - 24<br />43 - 47<br />47 - 59 | |19 - 24<br />43 - 47<br />47 - 59 | ||
| − | |7.1 - 9.1 | + | |7.1 - 9.1<sup>a</sup><br />3.7 - 4.0<sup>b</sup><br />2.9<sup>c</sup> |
|210 | |210 | ||
|18 | |18 | ||
| − | |131 | + | |131<sup>a</sup><br />138<sup>b</sup> |
|ca. 450 | |ca. 450 | ||
|1030 - 1070 | |1030 - 1070 | ||
| Line 128: | Line 139: | ||
|Ni 1.4 - 2.2<br />Be 0.2 - 0.6<br />Co 0.3<br />Cu Rest | |Ni 1.4 - 2.2<br />Be 0.2 - 0.6<br />Co 0.3<br />Cu Rest | ||
|8.8 | |8.8 | ||
| − | |11 - 14 | + | |11 - 14<sup>a</sup><br />25 - 27<sup>b</sup><br />27 - 34<sup>c</sup> |
|19 - 24<br />43 - 47<br />47 - 59 | |19 - 24<br />43 - 47<br />47 - 59 | ||
| − | |7.1 - 9.1 | + | |7.1 - 9.1<sup>a</sup><br />3.7 - 4.0<sup>b</sup><br />2.9<sup>c</sup> |
|230 | |230 | ||
|18 | |18 | ||
| − | |131 | + | |131<sup>a</sup><br />138<sup>b</sup> |
|ca. 480 | |ca. 480 | ||
|1060 - 1100 | |1060 - 1100 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
</figtable> | </figtable> | ||
| − | <br/> | + | |
| − | <br/> | + | <sup>a</sup>solution annealed, and cold rolled<br /> |
| + | <sup>b</sup>solution annealed, cold rolled, and precipitation hardened<br /> | ||
| + | <sup>c</sup>solution annealed, cold rolled, and precipitation hardened at mill (mill hardened) | ||
<figtable id="tab:Mechanical Properties of Selected Copper-Beryllium Alloys"> | <figtable id="tab:Mechanical Properties of Selected Copper-Beryllium Alloys"> | ||
| − | <caption>'''<!--Table 5.18:--> | + | <caption>'''<!--Table 5.18:-->Mechanical Properties of Selected Copper-Beryllium Alloys'''</caption> |
{| class="twocolortable" style="text-align: left; font-size: 12px" | {| class="twocolortable" style="text-align: left; font-size: 12px" | ||
|- | |- | ||
| − | ! | + | !Material |
| − | ! | + | !Hardness<br />Condition |
| − | ! | + | !Tensile Strength R<sub>m</sub><br />[MPa] |
| − | !0,2% | + | !0,2% Yield Strength<br />R<sub>p02</sub><br />[MPa] |
| − | ! | + | !Elongation<br />A<sub>50</sub><br />[%] |
| − | ! | + | !Vickers<br />Hardness<br />HV |
| − | ! | + | !Bend Radius<sup>1)</sup><br />perpendicular to<br />rolling direction |
| − | ! | + | !Bend Radius<sup>1)</sup><br />parallel to<br />rolling direction |
| − | ! | + | !Spring Bending<br />Limit σ<sub>FB</sub><br />[MPa] |
| − | ! | + | !Spring Fatigue<br />Limit σ<sub>BW</sub><br />[MPa] |
|- | |- | ||
|CuBe1,7 | |CuBe1,7 | ||
| − | |R 390 | + | |R 390<sup>a</sup><br />R 680<sup>a</sup><br />R 1030<sup>b</sup><br />R 1240<sup>b</sup><br />R 680<sup>c</sup><br />R 1100<sup>c</sup> |
|380 -520<br />680 - 820<br />1030 - 1240<br />1240 - 1380<br />680 - 750<br />1100 - 1200 | |380 -520<br />680 - 820<br />1030 - 1240<br />1240 - 1380<br />680 - 750<br />1100 - 1200 | ||
|≥ 180<br />≥ 600<br />≥ 900<br />≥ 1070<br />≥ 480<br />≥ 930 | |≥ 180<br />≥ 600<br />≥ 900<br />≥ 1070<br />≥ 480<br />≥ 930 | ||
| Line 173: | Line 183: | ||
|- | |- | ||
|CuBe2 | |CuBe2 | ||
| − | |R 410 | + | |R 410<sup>a</sup><br />R 690<sup>a</sup><br />R 1140<sup>b</sup><br />R 1310<sup>b</sup><br />R 690<sup>c</sup><br />R 1200<sup>c</sup> |
|410 -540<br />690 - 820<br />1140 - 1310<br />1310 - 1480<br />690 - 760<br />1200 - 1320 | |410 -540<br />690 - 820<br />1140 - 1310<br />1310 - 1480<br />690 - 760<br />1200 - 1320 | ||
|≥ 190<br />≥ 650<br />≥ 1000<br />≥ 1150<br />≥ 480<br />≥ 1030 | |≥ 190<br />≥ 650<br />≥ 1000<br />≥ 1150<br />≥ 480<br />≥ 1030 | ||
| Line 184: | Line 194: | ||
|- | |- | ||
|CuCo2Be<br />CuNi2Be | |CuCo2Be<br />CuNi2Be | ||
| − | |R 250 | + | |R 250<sup>a</sup><br />R 550<sup>a</sup><br />R 650<sup>b</sup><br />R 850<sup>b</sup><br />R 520<sup>c</sup> |
|250 - 380<br />550 - 700<br />650 - 820<br />850 - 1000<br />520 - 620 | |250 - 380<br />550 - 700<br />650 - 820<br />850 - 1000<br />520 - 620 | ||
|≥ 140<br />≥ 450<br />≥ 520<br />≥ 750<br />≥ 340 | |≥ 140<br />≥ 450<br />≥ 520<br />≥ 750<br />≥ 340 | ||
| Line 194: | Line 204: | ||
| <br /> <br />220<br />250<br />210 | | <br /> <br />220<br />250<br />210 | ||
|} | |} | ||
| − | < | + | </figtable> |
| − | < | + | <sup>1)</sup> t: Strip thickness max. 0.5 mm<br /> |
| − | < | + | <sup>a</sup>solution annealed, and cold rolled<br /> |
| − | < | + | <sup>b</sup>solution annealed, cold rolled, and precipitation hardened<br /> |
| − | < | + | <sup>c</sup>solution annealed, cold rolled, and precipitation hardened at mill (mill hardened) |
| − | + | ||
| − | < | + | ====<!--5.1.6.2-->Other Precipitation Hardening Copper Alloys==== |
| + | |||
| + | =====<!--5.1.6.2.1-->Copper-Chromium Alloys===== | ||
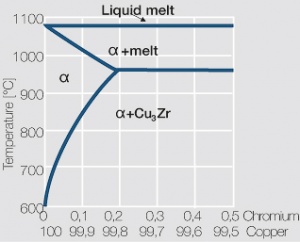
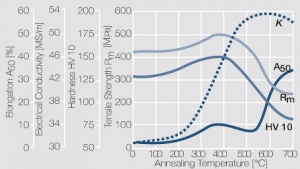
| − | = | + | As the phase diagram shows, copper-chromium has a similar hardening profile compared to CuBe <xr id="fig:Copper corner of the copper-chromium phase diagram for up to 0.8 wt% chromium"/><!--(Fig. 5.32)-->. In the hardened stage CuCr has limitations to work hardening. Compared to copper it has a better temperature stability with good electrical conductivity. Hardness and electrical conductivity as a function of cold working and precipitation hardening conditions are illustrated in [[#figures8|(Figs. 6 – 9)]]<!--Figs. 5.33-5.35-->, <xr id="tab:Physical Properties of Other Precipitation Hardening Copper Alloys"/><!--(Tables 5.19)--> and <xr id="tab:Mechanical Properties of Other Precipitation Hardening Copper Alloys"/><!--(Tab. 5.20)-->. |
| − | + | Copper-chromium materials are especially suitable for use as electrodes for resistance welding. During brazing the loss in hardness is limited if low melting brazing alloys and reasonably short heating times are used. | |
| − | + | <div id="figures5"> | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | <xr id="fig:Copper corner of the copper-chromium phase diagram for up to 0.8 wt% chromium"/><!--Fig. 5.32:--> Copper corner of the copper-chromium phase diagram for up to 0.8 wt% chromium | |
| − | + | ||
| − | + | <xr id="fig:Softening of precipitation hardened and subsequently cold worked CuCr1"/><!--Fig. 5.33:--> Softening of precipitation-hardened and subsequently cold worked CuCr1 after 4hrs annealing | |
| + | |||
| + | <xr id="fig:Electrical conductivity of precipitation hardened CuCr 0.6"/><!--Fig. 5.34 a:--> Electrical conductivity of precipitation hardened CuCr 0.6 as a function of annealing conditions | ||
| + | |||
| + | <xr id="fig:Hardness of precipitation hardened CuCr 0.6"/><!--Fig. 5.34 b:--> Hardness of precipitation hardened CuCr 0.6 as a function of annealing conditions | ||
| + | |||
| + | <xr id="fig:Electrical conductivity and hardness of precipitation hardened CuCr 0.6"/><!--Fig. 5.35:--> Electrical conductivity and hardness of precipitation hardened CuCr 0.6 after cold working | ||
| + | </div> | ||
<div class="multiple-images"> | <div class="multiple-images"> | ||
<figure id="fig:Copper corner of the copper-chromium phase diagram for up to 0.8 wt% chromium"> | <figure id="fig:Copper corner of the copper-chromium phase diagram for up to 0.8 wt% chromium"> | ||
| − | [[File:Copper corner of the copper chromium phase diagram.jpg|left|thumb|<caption> | + | [[File:Copper corner of the copper chromium phase diagram.jpg|left|thumb|<caption>Copper corner of the copper-chromium phase diagram for up to 0.8 wt% chromium</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Softening of precipitation hardened and subsequently cold worked CuCr1"> | <figure id="fig:Softening of precipitation hardened and subsequently cold worked CuCr1"> | ||
| − | [[File:Softening of precipitation hardened and subsequently cold worked CuCr1.jpg|left|thumb|<caption> | + | [[File:Softening of precipitation hardened and subsequently cold worked CuCr1.jpg|left|thumb|<caption>Softening of precipitation-hardened and subsequently cold worked CuCr1 after 4hrs annealing</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Electrical conductivity of precipitation hardened CuCr 0.6"> | <figure id="fig:Electrical conductivity of precipitation hardened CuCr 0.6"> | ||
| − | [[File:Electrical conductivity of precipitation hardened CuCr 0.6.jpg|left|thumb|<caption> | + | [[File:Electrical conductivity of precipitation hardened CuCr 0.6.jpg|left|thumb|<caption>Electrical conductivity of precipitation hardened CuCr 0.6 as a function of annealing conditions</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Hardness of precipitation hardened CuCr 0.6"> | <figure id="fig:Hardness of precipitation hardened CuCr 0.6"> | ||
| − | [[File:Hardness of precipitation hardened CuCr 0.6.jpg|left|thumb|<caption> | + | [[File:Hardness of precipitation hardened CuCr 0.6.jpg|left|thumb|<caption>Hardness of precipitation hardened CuCr 0.6 as a function of annealing conditions</caption>]] |
</figure> | </figure> | ||
<figure id="fig:Electrical conductivity and hardness of precipitation hardened CuCr 0.6"> | <figure id="fig:Electrical conductivity and hardness of precipitation hardened CuCr 0.6"> | ||
| − | [[File:Electrical conductivity and hardness of precipitation hardened CuCr 0.6.jpg|left|thumb|<caption> | + | [[File:Electrical conductivity and hardness of precipitation hardened CuCr 0.6.jpg|left|thumb|<caption>Electrical conductivity and hardness of precipitation hardened CuCr 0.6 after cold working</caption>]] |
</figure> | </figure> | ||
</div> | </div> | ||
| Line 243: | Line 257: | ||
<figtable id="tab:Physical Properties of Other Precipitation Hardening Copper Alloys"> | <figtable id="tab:Physical Properties of Other Precipitation Hardening Copper Alloys"> | ||
| − | <caption>'''<!--Table 5.19:--> | + | <caption>'''<!--Table 5.19:-->Physical Properties of Other Precipitation Hardening Copper Alloys'''</caption> |
{| class="twocolortable" style="text-align: left; font-size: 12px" | {| class="twocolortable" style="text-align: left; font-size: 12px" | ||
|- | |- | ||
| − | ! | + | !Material<br />Designation<br />EN UNS |
| − | ! | + | !Composition<br />[wt%] |
| − | ! | + | !Density<br />[g/cm<sup>3</sup>] |
| − | !colspan="2" style="text-align:center"| | + | !colspan="2" style="text-align:center"|Electrical<br />Conductivity |
| − | ! | + | !Electrical<br />Resistivity<br />[μΩ·cm] |
| − | ! | + | !Thermal<br />Conductivity<br />[W/(m·K)] |
| − | ! | + | !Coeff. of Linear<br />Thermal<br />Expansion<br />[10<sup>-6</sup>/K] |
| − | ! | + | !Modulus of<br />Elasticity<br />[GPa] |
| − | ! | + | !Softening Temperature<br />(approx. 10% loss in<br />strength)<br />[°C] |
| − | ! | + | !Melting<br />Temp Range<br />[°C] |
|- | |- | ||
! | ! | ||
| Line 273: | Line 287: | ||
|Cr 0.3 - 1.2<br />Cu Rest | |Cr 0.3 - 1.2<br />Cu Rest | ||
|8.89 | |8.89 | ||
| − | |26 | + | |26<sup>a</sup><br />48<sup>b</sup> |
| − | |45 | + | |45<sup>a</sup><br />83<sup>b</sup> |
| − | |3.8 | + | |3.8<sup>a</sup><br />2.1<sup>b</sup> |
| − | |170 | + | |170<sup>a</sup><br />315<sup>b</sup> |
|17 | |17 | ||
|112 | |112 | ||
| Line 285: | Line 299: | ||
|Zr 0.1 - 0.3<br />Cu Rest | |Zr 0.1 - 0.3<br />Cu Rest | ||
|8.9 | |8.9 | ||
| − | |35 | + | |35<sup>a</sup><br />52<sup>b</sup> |
| − | |60 | + | |60<sup>a</sup><br />90<sup>b</sup> |
| − | |2.9 | + | |2.9<sup>a</sup><br />1.9<sup>b</sup> |
| − | |340 | + | |340<sup>a</sup> |
|16 | |16 | ||
|135 | |135 | ||
| Line 297: | Line 311: | ||
|Cr 0.5 - 1.2<br />Zr 0.03 - 0.3<br />Cu Rest | |Cr 0.5 - 1.2<br />Zr 0.03 - 0.3<br />Cu Rest | ||
|8.92 | |8.92 | ||
| − | |20 | + | |20<sup>a</sup><br />43<sup>b</sup> |
| − | |34 | + | |34<sup>a</sup><br />74<sup>b</sup> |
| − | |5.0 | + | |5.0<sup>a</sup><br />2.3<sup>b</sup> |
| − | |170 | + | |170<sup>a</sup><br />310 - 330<sup>b</sup> |
|16 | |16 | ||
| − | |110<sup>a</sup><br />130 | + | |110<sup>a</sup><br />130<sup>b</sup> |
|ca. 500 | |ca. 500 | ||
|1070 - 1080 | |1070 - 1080 | ||
|} | |} | ||
| − | |||
| − | |||
</figtable> | </figtable> | ||
| − | <br /> | + | |
| − | <br /> | + | <sup>a</sup>solution annealed, and cold rolled<br /> |
| + | <sup>b</sup>solution annealed, cold rolled, and precipitation hardened<br /> | ||
<figtable id="tab:Mechanical Properties of Other Precipitation Hardening Copper Alloys"> | <figtable id="tab:Mechanical Properties of Other Precipitation Hardening Copper Alloys"> | ||
| − | <caption>'''<!--Table 5.20:--> | + | <caption>'''<!--Table 5.20:-->Mechanical Properties of Other Precipitation Hardening Copper Alloys'''</caption> |
<table class="twocolortable"> | <table class="twocolortable"> | ||
| − | <tr><th><p class="s16"> | + | <tr><th><p class="s16">Material</p></th><th><p class="s16">Hardness</p><p class="s16">Condi- tion</p></th><th><p class="s16">Tensile</p><p class="s16">Strength R<span class="s18">m</span></p><p class="s16">[MPa]</p></th><th><p class="s16">0,2% Yield</p><p class="s16">Strength R<span class="s18">p02</span></p><p class="s16">[MPa]</p></th><th><p class="s16">Elongation</p><p class="s16">A50</p><p class="s16">[%]</p></th><th><p class="s16">Vickers</p><p class="s16">Hardness</p><p class="s16">HV</p></th><th><p class="s16">Spring Bending</p><p class="s16">Limit <span class="s19">F</span><span class="s18">FB </span>[MPa]</p></th></tr><tr><td><p class="s16">CuCr</p></td><td><p class="s16">R 230<span class="s18">a</span></p><p class="s16">R 400<span class="s18">a </span>R 450<span class="s18">b </span>R 550<span class="s18">b</span></p></td><td><p class="s33">><span class="s16"> 230</span></p><p class="s33">><span class="s16"> 400</span></p><p class="s33">><span class="s16"> 450</span></p><p class="s33">><span class="s16"> 550</span></p></td><td><p class="s33">><span class="s16"> 80</span></p><p class="s33">><span class="s16"> 295</span></p><p class="s33">><span class="s16"> 325</span></p><p class="s33">><span class="s16"> 440</span></p></td><td><p class="s16">30</p><p class="s16">10</p><p class="s16">10</p><p class="s16">8</p></td><td><p class="s33">><span class="s16"> 55</span></p><p class="s33">><span class="s16"> 120</span></p><p class="s33">><span class="s16"> 130</span></p><p class="s33">><span class="s16"> 150</span></p></td><td><p class="s16">350</p></td></tr><tr><td><p class="s16">CuZr</p></td><td><p class="s16">R 260<span class="s18">a</span></p><p class="s16">R 370<span class="s18">a </span>R 400<span class="s18">b </span>R 420<span class="s18">b</span></p></td><td><p class="s33">><span class="s16"> 260</span></p><p class="s33">><span class="s16"> 370</span></p><p class="s33">><span class="s16"> 400</span></p><p class="s33">><span class="s16"> 420</span></p></td><td><p class="s33">><span class="s16"> 100</span></p><p class="s33">><span class="s16"> 270</span></p><p class="s33">><span class="s16"> 280</span></p><p class="s33">><span class="s16"> 400</span></p></td><td><p class="s16">35</p><p class="s16">12</p><p class="s16">12</p><p class="s16">10</p></td><td><p class="s33">><span class="s16"> 55</span></p><p class="s33">><span class="s16"> 100</span></p><p class="s33">><span class="s16"> 105</span></p><p class="s33">><span class="s16"> 115</span></p></td><td><p class="s16">280</p></td></tr><tr><td><p class="s16">CuCr1Zr</p></td><td><p class="s16">R 200<span class="s18">a</span></p><p class="s16">R 400<span class="s18">b</span></p><p class="s16">R 450<span class="s18">b</span></p></td><td><p class="s33">><span class="s16"> 200</span></p><p class="s33">><span class="s16"> 400</span></p><p class="s33">><span class="s16"> 450</span></p></td><td><p class="s33">><span class="s16"> 60</span></p><p class="s33">><span class="s16"> 210</span></p><p class="s33">><span class="s16"> 360</span></p></td><td><p class="s16">30</p><p class="s16">12</p><p class="s16">10</p></td><td><p class="s33">><span class="s16"> 70</span></p><p class="s33">><span class="s16"> 140</span></p><p class="s33">><span class="s16"> 155</span></p></td><td><p class="s16">420</p></td></tr></table> |
</figtable> | </figtable> | ||
| − | =====<!--5.1.6.2.2--> | + | =====<!--5.1.6.2.2-->Copper-Zirconium Alloys===== |
| + | |||
| + | The solubility of Zirconium in copper is 0.15 wt% Zr at the eutectic temperature of 980°C <xr id="fig:Copper corner of the copper zirconium for up to 0.5-wt zirconium"/><!--(Fig. 5.36)-->. Copper-zirconium materials have a similar properties spectrum compared to the one for copper-chromium materials. At room temperature the mechanical properties of copper-zirconium are less suitable than those of copper chromium, its temperature stability is however at least the same. | ||
| + | |||
| + | =====<!--5.1.6.2.3-->Copper-Chromium-Zirconium Alloys===== | ||
| − | + | The earlier used CuCr and CuZr materials have been partially replaced over the years by the capitation hardening three materials alloy CuCr1Zr. This material exhibits high mechanical strength at elevated temperatures and good oxidation resistance as well as high softening temperatures. In its hardened condition CuCr1Zr has also a high electrical conductivity <xr id="fig:Softening of CuCr1Zr after 1hr annealing"/><!--(Bild 5.37)-->. Their usage extends from mechanically and thermally highly stressed parts such as contact tulips in high voltage switchgear to electrodes for resistance welding. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | = | + | <xr id="fig:Copper corner of the copper zirconium for up to 0.5-wt zirconium"/><!--Fig. 5.36:--> Copper corner of the copper- zirconium for up to 0.5 wt% zirconium |
| − | + | <xr id="fig:Softening of CuCr1Zr after 1hr annealing"/><!--Fig. 5.37:--> Softening of CuCr1Zr after 1 hr annealing and after 90% cold working | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<div class="multiple-images"> | <div class="multiple-images"> | ||
<figure id="fig:Copper corner of the copper zirconium for up to 0.5-wt zirconium"> | <figure id="fig:Copper corner of the copper zirconium for up to 0.5-wt zirconium"> | ||
| − | [[File:Copper corner of the copper zirconium for up to 0.5-wt zirconium.jpg|right|thumb| | + | [[File:Copper corner of the copper zirconium for up to 0.5-wt zirconium.jpg|right|thumb|Copper corner of the copper- zirconium for up to 0.5 wt% zirconium]] |
</figure> | </figure> | ||
<figure id="fig:Softening of CuCr1Zr after 1hr annealing"> | <figure id="fig:Softening of CuCr1Zr after 1hr annealing"> | ||
| − | [[File:Softening of CuCr1Zr after 1hr annealing.jpg|right|thumb| | + | [[File:Softening of CuCr1Zr after 1hr annealing.jpg|right|thumb|Softening of CuCr1Zr after 1 hr annealing and after 90% cold working]] |
</figure> | </figure> | ||
</div> | </div> | ||
Revision as of 13:43, 20 September 2014
Neben den naturharten Kupferwerkstoffen spielen aushärtbare Kupferlegierungen als Trägerwerkstoffe für elektrische Kontakte eine wichtige Rolle. Bei den aushärtbaren Legierungen können durch eine geeignete Wärmebehandlung fein verteilte Ausscheidungen einer zweiten Phase erzeugt werden, die die Festigkeit des Werkstoffes deutlich erhöhen.
Contents
Kupfer-Beryllium-Legierungen (Berylliumbronze)
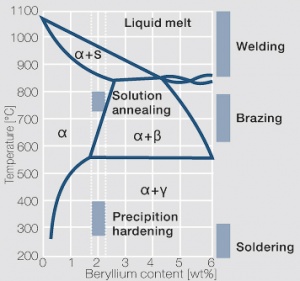
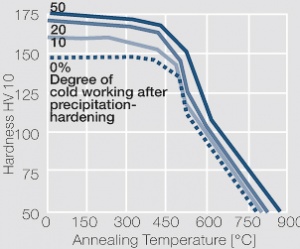
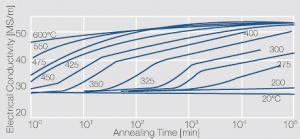
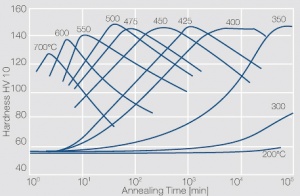
Voraussetzung für die Aushärtbarkeit der CuBe-Werkstoffe ist die mit sinkender Temperatur rasch abnehmende Löslichkeit des Berylliums im Kupfer. Wie aus dem Zustandsschaubild für CuBe ersichtlich, sind bei ca. 780°C 2,4 Massen-% Be in Kupfer löslich Figure 1. In diesem Temperaturbereich wärmebehandelte CuBe-Legierungen sind homogen („lösungsglühen“). Der homogene Zustand kann durch schnelles Abkühlen auf Raumtemperatur eingefroren werden („abschrecken“). Durch die anschließende Wärmebehandlung bei einer Temperatur von 325°C wird die gewünschte Ausscheidungshärtung erreicht, die einen deutlichen Anstieg der Festigkeit und der elektrischen Leitfähigkeit von CuBe bewirkt Table 1. Die erreichbaren Festigkeits- und Härtewerte sind abhängig von der Glühtemperatur und Glühdauer sowie vom Umformgrad (Tab. 5.18) und Table 2 and (Figs. 43 – 75).
Von den aushärtbaren Kupferlegierungen haben CuBe-Werkstoffe, vor allem
CuBe2 und CuBe1,7 für stromführende Kontaktfedern wegen ihrer herausragenden
mechanischen Eigenschaften eine besondere Bedeutung erlangt.
Daneben sind noch die Werkstoffe CuCo2Be und CuNi2Be zu erwähnen, die
bei mittleren Festigkeitswerten eine relativ hohe elektrische Leitfähigkeit
aufweisen.
CuBe-Legierungen sind als aushärtbare Halbzeuge in verschiedenen Lieferzuständen
erhältlich. Daneben können CuBe-Halbzeuge mit geringen Einbußen
hinsichtlich der Festigkeitseigenschaften im „werksvergüteten“ Zustand eingesetzt
werden. In diesem Falle wurde das Halbzeug bereits beim Hersteller
ausscheidungsgehärtet.
Da Beryllium in der EU-67/548 als kanzerogen eingestuft ist, wird bei einer Reihe von Legierungen versucht, das Eigenschaftsspektrum der bewährten CuBe1,7- und CuBe2-Werkstoffe wenigstens näherungsweise auch mit einem geringeren Be-Anteil zu erreichen. Auch die Entwicklung weiterer ausscheidungshärtender Kupfer-Legierungen ohne toxische bzw. deklarationspfichtige Elemente z.B. CuNiCoSi zielt in Richtung CuBe-Ersatz.
Figure 1 Zustandsdiagramm Kupfer-Beryllium mit Temperaturbereichen für Hartlötungen und Wärmebehandlungen
Figure 2 Aushärtung von CuBe2 bei 325°C nach unterschiedlicher Kaltumformung
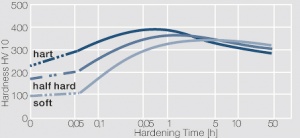
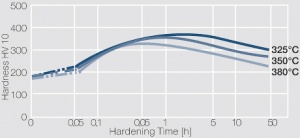
Figure 3 Precipitation hardening of CuBe2 (soft) at 325°C
Figure 4 Precipitation hardening of CuBe2 (half hard) at different annealing temperatures
| Material Designation EN UNS |
Composition [wt%] |
Density [g/cm3] |
Electrical Conductivity |
Electrical Resistivity [μΩ·cm] |
Thermal Conductivity [W/(m·K)] |
Coeff. of Linear Thermal Expansion [10-6/K] |
Modulus of Elasticity [GPa] |
Softening Temperature (approx. 10% loss in strength) [°C] |
Melting Temp Range [°C] | |
|---|---|---|---|---|---|---|---|---|---|---|
| [MS/m] | [% IACS] | |||||||||
| CuBe1.7 CW100C C17000 |
Be 1.6 - 1.8 Co 0.3 Ni 0.3 Cu Rest |
8.4 | 8 - 9a 12 - 13b 11c |
14 - 16 21 - 22 19 |
11 - 12.5a 7.7 - 8.3b 9.1c |
110 | 17 | 125a 135b |
ca. 380 | 890 - 1000 |
| CuBe2 CW101C C17200 |
Be 1.8 - 2.1 Co 0.3 Ni 0.3 Cu Rest |
8.3 | 8 - 9a 12 - 13b 11c |
14 - 16 21 - 22 19 |
11 - 12.5a 7.7 - 8.3b 9.1c |
110 | 17 | 125a 135b |
ca. 380 | 870 - 980 |
| CuCo2Be CW104C C17500 |
Co 2.0 - 2.8 Be 0.4 - 0.7 Ni 0.3 Cu Rest |
8.8 | 11 - 14a 25 - 27b 27 - 34c |
19 - 24 43 - 47 47 - 59 |
7.1 - 9.1a 3.7 - 4.0b 2.9c |
210 | 18 | 131a 138b |
ca. 450 | 1030 - 1070 |
| CuNi2Be CW110C C17510 |
Ni 1.4 - 2.2 Be 0.2 - 0.6 Co 0.3 Cu Rest |
8.8 | 11 - 14a 25 - 27b 27 - 34c |
19 - 24 43 - 47 47 - 59 |
7.1 - 9.1a 3.7 - 4.0b 2.9c |
230 | 18 | 131a 138b |
ca. 480 | 1060 - 1100 |
asolution annealed, and cold rolled
bsolution annealed, cold rolled, and precipitation hardened
csolution annealed, cold rolled, and precipitation hardened at mill (mill hardened)
| Material | Hardness Condition |
Tensile Strength Rm [MPa] |
0,2% Yield Strength Rp02 [MPa] |
Elongation A50 [%] |
Vickers Hardness HV |
Bend Radius1) perpendicular to rolling direction |
Bend Radius1) parallel to rolling direction |
Spring Bending Limit σFB [MPa] |
Spring Fatigue Limit σBW [MPa] |
|---|---|---|---|---|---|---|---|---|---|
| CuBe1,7 | R 390a R 680a R 1030b R 1240b R 680c R 1100c |
380 -520 680 - 820 1030 - 1240 1240 - 1380 680 - 750 1100 - 1200 |
≥ 180 ≥ 600 ≥ 900 ≥ 1070 ≥ 480 ≥ 930 |
35 2 3 1 18 3 |
80 - 135 210 - 250 330 - 380 360 - 420 220 - 350 330 - 370 |
0 x t 1 x t 1 x t 1 x t 6 x t |
0 x t 3 x t 1.5 x t 1 x t 10 x t |
700 1000 390 790 |
260 280 260 |
| CuBe2 | R 410a R 690a R 1140b R 1310b R 690c R 1200c |
410 -540 690 - 820 1140 - 1310 1310 - 1480 690 - 760 1200 - 1320 |
≥ 190 ≥ 650 ≥ 1000 ≥ 1150 ≥ 480 ≥ 1030 |
35 2 3 1 18 3 |
90 - 140 215 - 260 350 - 400 380 - 450 220 - 250 360 - 410 |
0 x t 1 x t 1 x t 5 x t |
0 x t 3 x t 1.5 x t 10 x t |
800 1040 400 900 |
270 300 280 |
| CuCo2Be CuNi2Be |
R 250a R 550a R 650b R 850b R 520c |
250 - 380 550 - 700 650 - 820 850 - 1000 520 - 620 |
≥ 140 ≥ 450 ≥ 520 ≥ 750 ≥ 340 |
20 2 10 1 5 |
60 - 90 160 - 200 195 - 230 240 - 290 150 - 180 |
0 x t 3 x t 1 x t 3 x t 1 x t |
0 x t 1 x t 3.5 x t 1 x t |
360 650 300 |
220 250 210 |
1) t: Strip thickness max. 0.5 mm
asolution annealed, and cold rolled
bsolution annealed, cold rolled, and precipitation hardened
csolution annealed, cold rolled, and precipitation hardened at mill (mill hardened)
Other Precipitation Hardening Copper Alloys
Copper-Chromium Alloys
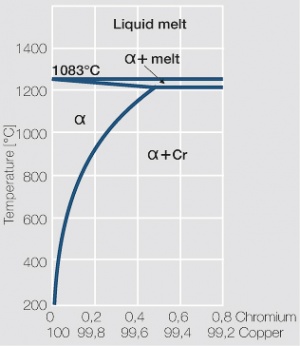
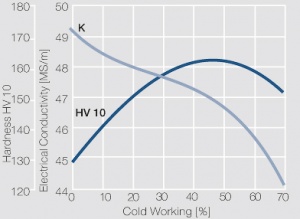
As the phase diagram shows, copper-chromium has a similar hardening profile compared to CuBe Figure 5. In the hardened stage CuCr has limitations to work hardening. Compared to copper it has a better temperature stability with good electrical conductivity. Hardness and electrical conductivity as a function of cold working and precipitation hardening conditions are illustrated in (Figs. 6 – 9), Table 3 and Table 4.
Copper-chromium materials are especially suitable for use as electrodes for resistance welding. During brazing the loss in hardness is limited if low melting brazing alloys and reasonably short heating times are used.
Figure 5 Copper corner of the copper-chromium phase diagram for up to 0.8 wt% chromium
Figure 6 Softening of precipitation-hardened and subsequently cold worked CuCr1 after 4hrs annealing
Figure 7 Electrical conductivity of precipitation hardened CuCr 0.6 as a function of annealing conditions
Figure 8 Hardness of precipitation hardened CuCr 0.6 as a function of annealing conditions
Figure 9 Electrical conductivity and hardness of precipitation hardened CuCr 0.6 after cold working
| Material Designation EN UNS |
Composition [wt%] |
Density [g/cm3] |
Electrical Conductivity |
Electrical Resistivity [μΩ·cm] |
Thermal Conductivity [W/(m·K)] |
Coeff. of Linear Thermal Expansion [10-6/K] |
Modulus of Elasticity [GPa] |
Softening Temperature (approx. 10% loss in strength) [°C] |
Melting Temp Range [°C] | |
|---|---|---|---|---|---|---|---|---|---|---|
| [MS/m] | [% IACS] | |||||||||
| CuCr | Cr 0.3 - 1.2 Cu Rest |
8.89 | 26a 48b |
45a 83b |
3.8a 2.1b |
170a 315b |
17 | 112 | ca. 450 | 980 - 1080 |
| CuZr | Zr 0.1 - 0.3 Cu Rest |
8.9 | 35a 52b |
60a 90b |
2.9a 1.9b |
340a | 16 | 135 | ca. 500 | 1020 - 1080 |
| CuCr1Zr CW106C C18150 |
Cr 0.5 - 1.2 Zr 0.03 - 0.3 Cu Rest |
8.92 | 20a 43b |
34a 74b |
5.0a 2.3b |
170a 310 - 330b |
16 | 110a 130b |
ca. 500 | 1070 - 1080 |
asolution annealed, and cold rolled
bsolution annealed, cold rolled, and precipitation hardened
Material | Hardness Condi- tion | Tensile Strength Rm [MPa] | 0,2% Yield Strength Rp02 [MPa] | Elongation A50 [%] | Vickers Hardness HV | Spring Bending Limit FFB [MPa] |
|---|---|---|---|---|---|---|
CuCr | R 230a R 400a R 450b R 550b | > 230 > 400 > 450 > 550 | > 80 > 295 > 325 > 440 | 30 10 10 8 | > 55 > 120 > 130 > 150 | 350 |
CuZr | R 260a R 370a R 400b R 420b | > 260 > 370 > 400 > 420 | > 100 > 270 > 280 > 400 | 35 12 12 10 | > 55 > 100 > 105 > 115 | 280 |
CuCr1Zr | R 200a R 400b R 450b | > 200 > 400 > 450 | > 60 > 210 > 360 | 30 12 10 | > 70 > 140 > 155 | 420 |
Copper-Zirconium Alloys
The solubility of Zirconium in copper is 0.15 wt% Zr at the eutectic temperature of 980°C Figure 10. Copper-zirconium materials have a similar properties spectrum compared to the one for copper-chromium materials. At room temperature the mechanical properties of copper-zirconium are less suitable than those of copper chromium, its temperature stability is however at least the same.
Copper-Chromium-Zirconium Alloys
The earlier used CuCr and CuZr materials have been partially replaced over the years by the capitation hardening three materials alloy CuCr1Zr. This material exhibits high mechanical strength at elevated temperatures and good oxidation resistance as well as high softening temperatures. In its hardened condition CuCr1Zr has also a high electrical conductivity Figure 11. Their usage extends from mechanically and thermally highly stressed parts such as contact tulips in high voltage switchgear to electrodes for resistance welding.
Figure 10 Copper corner of the copper- zirconium for up to 0.5 wt% zirconium
Figure 11 Softening of CuCr1Zr after 1 hr annealing and after 90% cold working