Difference between revisions of "Gold Based Materials"
Doduco Admin (talk | contribs) |
|||
| (18 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | Pure Gold is besides Platinum the chemically most stable of all precious metals. In its pure form it is not very suitable for use as a contact material in electromechanical devices because of its tendency to stick and cold-weld at even low contact forces. In addition it is not hard or strong enough to resist mechanical wear and exhibits high | + | Pure Gold is besides Platinum the chemically most stable of all precious metals. In its pure form it is not very suitable for use as a contact material in electromechanical devices because of its tendency to stick and cold-weld at even low contact forces. In addition it is not hard or strong enough to resist mechanical wear and exhibits high materials losses under electrical arcing loads <xr id="tab:Contact_and_Switching_Properties_of_Gold_and_Gold_Alloys"/> (Tab. 2.4). This limits its use in form of thin electroplated or vacuum deposited layers. |
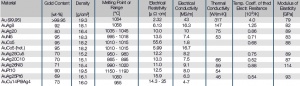
| − | For most electrical contact applications | + | For most electrical contact applications gold alloys are used. Depending on the alloying metal the melting is performed either under in a reducing atmosphere or in a vacuum. The choice of alloying metals depends on the intended use of the resulting contact material. The binary Au alloys with typically <10 wt% of other precious metals such as Pt, Pd, or Ag or non-precious metals like Ni, Co, and Cu are the more commonly used ones <xr id="tab:Physical Properties of Gold and Gold-Alloys"/> ''(Tab. 2.2)''. |
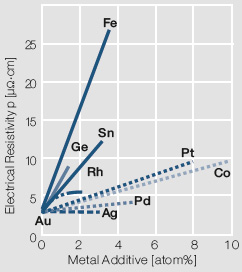
| − | On one hand these alloy additions improve the mechanical strength and electrical switching properties but on the other hand reduce the electrical conductivity and chemical corrosion resistance | + | On one hand these alloy additions improve the mechanical strength and electrical switching properties but on the other hand reduce the electrical conductivity and chemical corrosion resistance <xr id="fig:Influence of 1-10 atomic of different"/> ''(Fig. 2.2)'' to varying degrees. |
| − | Under the aspect of reducing the gold content | + | Under the aspect of reducing the gold content ternary alloys with a gold content of approximately 70 wt% and additions of Ag and Cu or Ag and Ni resp., for example AuAg25Cu5 or AuAg20Cu10 are used which exhibit for many applications good mechanical stability while at the same time have sufficient resistance against the formation of corrosion layers <xr id="tab:Mechanical Properties of Gold and Gold-Alloys"/> ''(Table 2.3)''. |
<figtable id="tab:Commonly Used Grades of Gold"> | <figtable id="tab:Commonly Used Grades of Gold"> | ||
| − | <caption>'''Commonly Used Grades of Gold | + | <caption>'''Tab: Commonly Used Grades of Gold (2.1)'''</caption> |
<table class="twocolortable"> | <table class="twocolortable"> | ||
| − | <tr><th><p class="s11">Designation</p></th><th><p class="s11">Composition Au</p><p class="s11">(min. content)</p></th><th><p class="s11">Impurites ppm</p></th><th><p class="s12">Remarks on forms and application</p></th></tr><tr><td><p class="s11">Electronic | + | <tr><th><p class="s11">Designation</p></th><th><p class="s11">Composition Au</p><p class="s11">(min. content)</p></th><th><p class="s11">Impurites ppm</p></th><th><p class="s12">Remarks on forms and application</p></th></tr><tr><td><p class="s11">Electronic Glod</p><p class="s11">Gold</p></td><td><p class="s11">99.999</p></td><td><p class="s11">Cu < 3</p><p class="s11">Ag < 3</p><p class="s11">Ca < 1</p><p class="s11">Mg <1</p><p class="s11">Fe < 1</p></td><td><p class="s12">Wires, strips, alloying metal for semiconductors, electronic components</p></td></tr><tr><td><p class="s11">Pure Gold</p></td><td><p class="s11">99.995</p></td><td><p class="s11">Cu < 10</p><p class="s11">Ag < 15</p><p class="s11">Ca < 20</p><p class="s11">Mg < 10</p><p class="s11">Fe < 3</p><p class="s11">Si < 10</p><p class="s11">Pb < 20</p></td><td><p class="s12">Granulate for high purity alloys, strips, tubing, profiles</p></td></tr><tr><td><p class="s11">Ingot Grade-Gold</p></td><td><p class="s11">99.95</p></td><td><p class="s11">Cu < 100</p><p class="s11">Ag < 150</p><p class="s11">Ca < 50</p><p class="s11">Mg < 50</p><p class="s11">Fe < 30</p><p class="s11">Si < 10</p></td><td><p class="s12">Alloys, commonly used grade</p></td></tr></table> |
</figtable> | </figtable> | ||
| − | |||
| − | |||
| + | <div class="multiple-images"> | ||
<figtable id="tab:Physical Properties of Gold and Gold-Alloys"> | <figtable id="tab:Physical Properties of Gold and Gold-Alloys"> | ||
| − | + | [[File:Physical Properties of Gold and Gold-Alloys.jpg|left|thumb|<caption>Tab 2.2 Physical Properties of Gold and Gold-Alloys</caption>]] | |
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
</figtable> | </figtable> | ||
| − | + | <figure id="fig:Influence of 1-10 atomic of different"> | |
| − | |||
| − | <figure id="fig: | ||
[[File:Influence of 1-10 atomic of different.jpg|left|thumb|<caption>Fig 2.2 Influence of 1-10 atomic% of different alloying metals on the electrical resistivity of gold (according to J. O. Linde)</caption>]] | [[File:Influence of 1-10 atomic of different.jpg|left|thumb|<caption>Fig 2.2 Influence of 1-10 atomic% of different alloying metals on the electrical resistivity of gold (according to J. O. Linde)</caption>]] | ||
</figure> | </figure> | ||
| Line 163: | Line 26: | ||
<figtable id="tab:Mechanical Properties of Gold and Gold-Alloys"> | <figtable id="tab:Mechanical Properties of Gold and Gold-Alloys"> | ||
| − | <caption>''' | + | <caption>'''Tab.2.3: Mechanical Properties of Gold and Gold-Alloys'''</caption> |
{| class="twocolortable" style="text-align: left; font-size: 12px" | {| class="twocolortable" style="text-align: left; font-size: 12px" | ||
|- | |- | ||
| Line 246: | Line 109: | ||
</figtable> | </figtable> | ||
| − | Other ternary alloys based on the AuAg system are AuAg26Ni3 and AuAg25Pt6. These alloys are mechanically similar to the AuAgCu alloys but have significantly higher oxidation resistance at elevated temperatures | + | Other ternary alloys based on the AuAg system are AuAg26Ni3 and AuAg25Pt6. These alloys are mechanically similar to the AuAgCu alloys but have significantly higher oxidation resistance at elevated temperatures <xr id="tab:Contact_and_Switching_Properties_of_Gold_and_Gold_Alloys"/>''(Table 2.4)''. |
<figtable id="tab:Contact_and_Switching_Properties_of_Gold_and_Gold_Alloys"> | <figtable id="tab:Contact_and_Switching_Properties_of_Gold_and_Gold_Alloys"> | ||
| − | <caption>''' | + | <caption>'''Table 2.4: Contact and Switching Properties of Gold and Gold Alloys'''</caption> |
<table class="twocolortable"> | <table class="twocolortable"> | ||
<tr><th><p class="s11">Material</p></th><th><p class="s12">Properties<th colspan="2"></p></th></tr><tr><td><p class="s11">Au</p></td><td><p class="s12">Highest corrosion resistance, low</p><p class="s12">hardness</p></td><td><p class="s12">High electr. conductivity,</p><p class="s12">strong tendency to cold welding</p></td></tr><tr><td><p class="s11">AuAg8</p></td><td><p class="s12">High corrosion resistance, low thermo</p><p class="s12">e.m.f.</p></td><td><p class="s12">Low contact resistance</p></td></tr><tr><td><p class="s11">AuPt10</p><p class="s11">AuPd5</p></td><td><p class="s12">Very high corrosion resistance</p></td><td><p class="s12">High hardness</p></td></tr><tr><td><p class="s11">AuAg10 - 30</p></td><td><p class="s12">Mostly corrosion resistant</p></td><td><p class="s12">Higher hardness</p></td></tr><tr><td><p class="s11">AuNi5</p><p class="s11">AuCo5</p></td><td><p class="s12">High corrosion resistance, low</p><p class="s12">tendency to material transfer</p></td><td><p class="s12">High hardness</p></td></tr><tr><td><p class="s11">AuAg25Pt6</p></td><td><p class="s12">High corrosion resistance, low contact resistance</p></td><td><p class="s12">High hardness</p></td></tr><tr><td><p class="s11">AuAg26Ni3</p><p class="s11">AuAg25Cu5</p><p class="s11">AuAg20Cu10</p></td><td><p class="s12">Limited corrosion resistance</p></td><td><p class="s12">High hardness</p></td></tr><tr><td><p class="s11">AuPd40</p><p class="s11">AuPd35Ag10</p><p class="s11">AuCu14Pt9Ag4</p></td><td><p class="s12">High corrosion resistance</p></td><td><p class="s12">High hardness and mechanical</p><p class="s12">wear resistance</p></td></tr></table> | <tr><th><p class="s11">Material</p></th><th><p class="s12">Properties<th colspan="2"></p></th></tr><tr><td><p class="s11">Au</p></td><td><p class="s12">Highest corrosion resistance, low</p><p class="s12">hardness</p></td><td><p class="s12">High electr. conductivity,</p><p class="s12">strong tendency to cold welding</p></td></tr><tr><td><p class="s11">AuAg8</p></td><td><p class="s12">High corrosion resistance, low thermo</p><p class="s12">e.m.f.</p></td><td><p class="s12">Low contact resistance</p></td></tr><tr><td><p class="s11">AuPt10</p><p class="s11">AuPd5</p></td><td><p class="s12">Very high corrosion resistance</p></td><td><p class="s12">High hardness</p></td></tr><tr><td><p class="s11">AuAg10 - 30</p></td><td><p class="s12">Mostly corrosion resistant</p></td><td><p class="s12">Higher hardness</p></td></tr><tr><td><p class="s11">AuNi5</p><p class="s11">AuCo5</p></td><td><p class="s12">High corrosion resistance, low</p><p class="s12">tendency to material transfer</p></td><td><p class="s12">High hardness</p></td></tr><tr><td><p class="s11">AuAg25Pt6</p></td><td><p class="s12">High corrosion resistance, low contact resistance</p></td><td><p class="s12">High hardness</p></td></tr><tr><td><p class="s11">AuAg26Ni3</p><p class="s11">AuAg25Cu5</p><p class="s11">AuAg20Cu10</p></td><td><p class="s12">Limited corrosion resistance</p></td><td><p class="s12">High hardness</p></td></tr><tr><td><p class="s11">AuPd40</p><p class="s11">AuPd35Ag10</p><p class="s11">AuCu14Pt9Ag4</p></td><td><p class="s12">High corrosion resistance</p></td><td><p class="s12">High hardness and mechanical</p><p class="s12">wear resistance</p></td></tr></table> | ||
</figtable> | </figtable> | ||
| − | Caused by higher gold prices over the past years | + | Caused by higher gold prices over the past years the development of alloys with further reduced gold content had a high priority. The starting point has been the AuPd system which has continuous solubility of the two components. Besides the binary alloy of AuPd40 and the ternary one AuPd35Ag9 other multiple component alloys were developed. These alloys typically have < 50 wt% Au and often can be solution hardened in order to obtain even higher hardness and tensile strength. They are mostly used in sliding contact applications. |
| − | Gold alloys are used in the form of welded wire or profile (also called weldtapes), segments, contact rivets and stampings produced from clad strip | + | Gold alloys are used in the form of welded wire or profile (also called weldtapes), segments, contact rivets, and stampings produced from clad strip |
| − | materials. The selection of the bonding process is based on the cost for the joining process and most importantly on the economical aspect of using the least possible amount of the expensive precious metal component. | + | materials. The selection of the bonding process is based on the cost for the joining process, and most importantly on the economical aspect of using the |
| + | least possible amount of the expensive precious metal component. | ||
| − | Besides being used as switching contacts in relays and pushbuttons, gold alloys are also applied in the design of connectors as well as sliding contacts for potentiometers, sensors, slip rings and brushes in miniature DC motors | + | Besides being used as switching contacts in relays and pushbuttons, gold alloys are also applied in the design of connectors as well as sliding contacts for potentiometers, sensors, slip rings, and brushes in miniature DC motors <xr id="tab:Application Examples and Forms of Gold and Gold Alloys"/>''(Table 2.5)''. |
<figtable id="tab:Application Examples and Forms of Gold and Gold Alloys"> | <figtable id="tab:Application Examples and Forms of Gold and Gold Alloys"> | ||
| − | <caption>''' | + | <caption>'''Table 2.5: Application Examples and Forms of Gold and Gold Alloys'''</caption> |
<table class="twocolortable"> | <table class="twocolortable"> | ||
<tr><th><p class="s11">Material</p></th><th><p class="s12">Application Examples</p></th><th><p class="s12">Form of Application</p></th></tr><tr><td><p class="s11">Pure Gold</p><p class="s11">(electroplated)</p></td><td><p class="s12">Corrosion protection layer for contact parts, stationary contacts, bonding surfaces</p></td><td><p class="s12">Electroplated coatings, bond surface layers</p></td></tr><tr><td><p class="s11">Hard Gold</p><p class="s11">(sputtered)</p></td><td><p class="s12">Contact parts for connectors and switches, sliding contact tracks, bonding surfaces</p></td><td><p class="s12">Electroplated coatings on contact rivets and stamped parts</p></td></tr><tr><td><p class="s11">Hard Gold</p><p class="s11">(sputtered)</p></td><td><p class="s12">Contacts in switches and relays for low loads, electronic signal relays</p></td><td><p class="s12">Contact surface layer on miniature</p><p class="s12">profiles (weld tapes)</p></td></tr><tr><td><p class="s11">AuAg8</p></td><td><p class="s12">Dry circuit switching contacts, electronic</p><p class="s12">signal relays</p></td><td><p class="s12">Contact rivets, welded contact</p><p class="s12">parts</p></td></tr><tr><td><p class="s11">AuAg20</p></td><td><p class="s12">Switching contacts for low loads, electronic</p><p class="s12">signal relays</p></td><td><p class="s12">Contact rivets, welded contact</p><p class="s12">parts</p></td></tr><tr><td><p class="s11">AuAg25Cu5</p><p class="s11">AuAg25Cu10</p><p class="s11">AuAg26Ni3</p></td><td><p class="s12">Contact parts for connectors, switches and relays</p></td><td><p class="s12">Claddings on Cu alloys, contact rivets, contact layer on micro profiles (weld tapes)</p></td></tr><tr><td><p class="s11">AuNi5</p><p class="s11">AuCo5 (heterogen)</p></td><td><p class="s12">Contacts in switches and relays for low and medium loads, material transfer resistant contacts</p></td><td><p class="s12">Contact rivets, welded contact parts, contact layer on miniature profiles (weld tapes)</p></td></tr><tr><td><p class="s11">AuPt10</p><p class="s11">AuAg25Pt6</p></td><td><p class="s12">Contacts for highest chemical corrosion resistance in switches and relays</p></td><td><p class="s12">Contact rivets, contact layer on micro profiles (weld tapes)</p></td></tr><tr><td><p class="s11">AuCu14Pt9Ag4</p></td><td><p class="s12">Sliding contacts for measurement data transfer</p></td><td><p class="s12">Wire-formed parts</p></td></tr></table> | <tr><th><p class="s11">Material</p></th><th><p class="s12">Application Examples</p></th><th><p class="s12">Form of Application</p></th></tr><tr><td><p class="s11">Pure Gold</p><p class="s11">(electroplated)</p></td><td><p class="s12">Corrosion protection layer for contact parts, stationary contacts, bonding surfaces</p></td><td><p class="s12">Electroplated coatings, bond surface layers</p></td></tr><tr><td><p class="s11">Hard Gold</p><p class="s11">(sputtered)</p></td><td><p class="s12">Contact parts for connectors and switches, sliding contact tracks, bonding surfaces</p></td><td><p class="s12">Electroplated coatings on contact rivets and stamped parts</p></td></tr><tr><td><p class="s11">Hard Gold</p><p class="s11">(sputtered)</p></td><td><p class="s12">Contacts in switches and relays for low loads, electronic signal relays</p></td><td><p class="s12">Contact surface layer on miniature</p><p class="s12">profiles (weld tapes)</p></td></tr><tr><td><p class="s11">AuAg8</p></td><td><p class="s12">Dry circuit switching contacts, electronic</p><p class="s12">signal relays</p></td><td><p class="s12">Contact rivets, welded contact</p><p class="s12">parts</p></td></tr><tr><td><p class="s11">AuAg20</p></td><td><p class="s12">Switching contacts for low loads, electronic</p><p class="s12">signal relays</p></td><td><p class="s12">Contact rivets, welded contact</p><p class="s12">parts</p></td></tr><tr><td><p class="s11">AuAg25Cu5</p><p class="s11">AuAg25Cu10</p><p class="s11">AuAg26Ni3</p></td><td><p class="s12">Contact parts for connectors, switches and relays</p></td><td><p class="s12">Claddings on Cu alloys, contact rivets, contact layer on micro profiles (weld tapes)</p></td></tr><tr><td><p class="s11">AuNi5</p><p class="s11">AuCo5 (heterogen)</p></td><td><p class="s12">Contacts in switches and relays for low and medium loads, material transfer resistant contacts</p></td><td><p class="s12">Contact rivets, welded contact parts, contact layer on miniature profiles (weld tapes)</p></td></tr><tr><td><p class="s11">AuPt10</p><p class="s11">AuAg25Pt6</p></td><td><p class="s12">Contacts for highest chemical corrosion resistance in switches and relays</p></td><td><p class="s12">Contact rivets, contact layer on micro profiles (weld tapes)</p></td></tr><tr><td><p class="s11">AuCu14Pt9Ag4</p></td><td><p class="s12">Sliding contacts for measurement data transfer</p></td><td><p class="s12">Wire-formed parts</p></td></tr></table> | ||
</figtable> | </figtable> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <xr id="fig:Phase diagram of goldplatinum"/> Fig. 2.3: Phase diagram of goldplatinum | ||
| + | |||
| + | |||
| + | <xr id="fig:Phase diagram of gold-silver"/> Fig. 2.4: Phase diagram of gold-silver | ||
| + | |||
| + | |||
| + | <xr id="fig:Phase diagram of gold-copper"/> Fig. 2.5: Phase diagram of gold-copper | ||
| + | |||
| + | |||
| + | <xr id="fig:Phase diagram of gold-nickel"/> Fig. 2.6: Phase diagram of gold-nickel | ||
| + | |||
| + | |||
| + | <xr id="fig:Phase diagram of gold-cobalt"/> Fig. 2.7: Phase diagram of gold-cobalt | ||
| + | |||
| + | |||
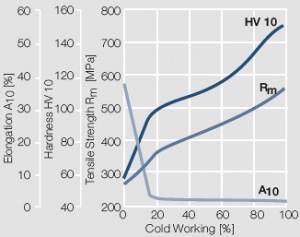
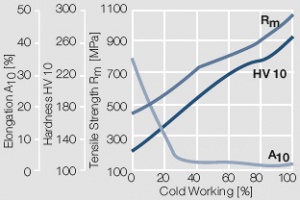
| + | <xr id="fig:Strain hardening of Au by cold working"/> Fig. 2.8: Strain hardening of Au by cold working | ||
| + | |||
| + | |||
| + | <xr id="fig:Softening of Au after annealing for 0.5 hrs"/> Fig. 2.9: Softening of Au after annealing for 0.5 hrs after 80% cold working | ||
| + | |||
| + | |||
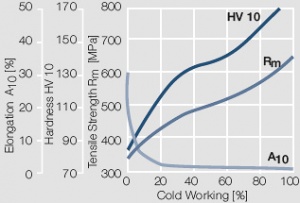
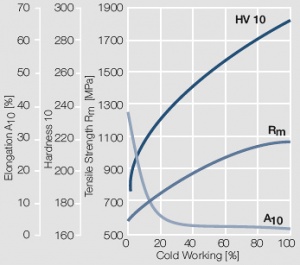
| + | <xr id="fig:Strain hardening of AuPt10 by cold working"/> Fig. 2.10: Strain hardening of AuPt10 by cold working | ||
| + | |||
| + | |||
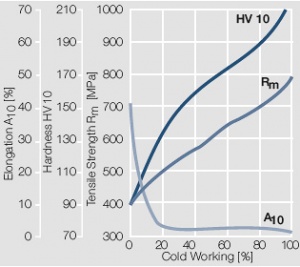
| + | <xr id="fig:Strain hardening of AuAg20 by cold working"/> Fig. 2.11: Strain hardening of AuAg20 by cold working | ||
| + | |||
| + | |||
| + | <xr id="fig:Strain hardening of AuAg30 by cold working"/> Fig. 2.12: Strain hardening of AuAg30 by cold working | ||
| + | |||
| + | |||
| + | <xr id="fig:Strain hardening of AuNi5 by cold working"/> Fig. 2.13: Strain hardening of AuNi5 by cold working | ||
| + | |||
| + | |||
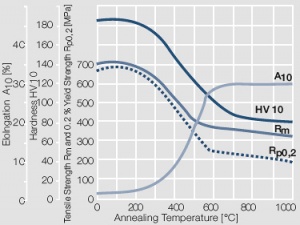
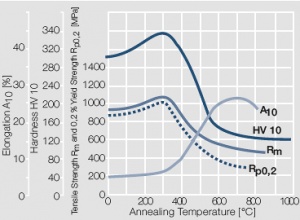
| + | <xr id="fig:Softening of AuNi5 after annealing for 0.5 hrs"/> Fig. 2.14: Softening of AuNi5 after annealing for 0.5 hrs after 80% cold working | ||
| + | |||
| + | |||
| + | <xr id="fig:Strain hardening of AuCo5 by cold working"/> Fig. 2.15: Strain hardening of AuCo5 by cold working | ||
| + | |||
| + | |||
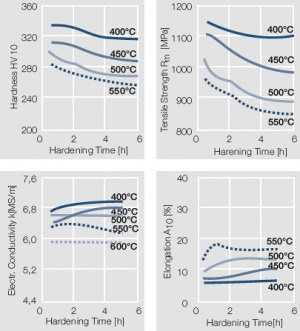
| + | <xr id="fig:Precipitation hardening of AuCo5 at"/> Fig. 2.16: Precipitation hardening of AuCo5 at 400°C hardening temperature | ||
| + | |||
| + | |||
| + | <xr id="fig:Strain hardening of AuAg25Pt6 by cold working"/> Fig. 2.17: Strain hardening of AuAg25Pt6 by cold working | ||
| + | |||
| + | |||
| + | <xr id="fig:Strain hardening of AuAg26Ni3 by cold working"/> Fig. 2.18: Strain hardening of AuAg26Ni3 by cold working | ||
| + | |||
| + | |||
| + | <xr id="fig:Softening of AuAg26Ni3 after annealing for 0.5-hrs"/> Fig. 2.19: Softening of AuAg26Ni3 after annealing for 0.5 hrs after 80% cold working | ||
| + | |||
| + | |||
| + | <xr id="fig:Strain hardening of AuAg25Cu5 by cold working"/> Fig. 2.20: Strain hardening of AuAg25Cu5 by cold working | ||
| + | |||
| + | |||
| + | <xr id="fig:Strain hardening of AuAg20Cu10 by cold working"/> Fig. 2.21: Strain hardening of AuAg20Cu10 by cold working | ||
| + | |||
| + | |||
| + | <xr id="fig:Softening of AuAg20Cu10 after annealing for 0.5 hrs"/> Fig. 2.22: Softening of AuAg20Cu10 after annealing for 0.5 hrs after 80% cold working | ||
| + | |||
| + | |||
| + | <xr id="fig:Strain hardening of AuCu14Pt9Ag4 by cold working"/> Fig. 2.23: Strain hardening of AuCu14Pt9Ag4 by cold working | ||
| + | |||
| + | |||
| + | <xr id="fig:Precipitation hardening of AuCu14Pt9Ag4"/> Fig. 2.24: Precipitation hardening of AuCu14Pt9Ag4 at different hardening temperatures after 50% cold working | ||
| + | |||
| Line 362: | Line 295: | ||
==References== | ==References== | ||
[[Contact Materials for Electrical Engineering#References|References]] | [[Contact Materials for Electrical Engineering#References|References]] | ||
| − | |||
| − | |||
Revision as of 12:19, 14 May 2014
Pure Gold is besides Platinum the chemically most stable of all precious metals. In its pure form it is not very suitable for use as a contact material in electromechanical devices because of its tendency to stick and cold-weld at even low contact forces. In addition it is not hard or strong enough to resist mechanical wear and exhibits high materials losses under electrical arcing loads Table 4 (Tab. 2.4). This limits its use in form of thin electroplated or vacuum deposited layers.
For most electrical contact applications gold alloys are used. Depending on the alloying metal the melting is performed either under in a reducing atmosphere or in a vacuum. The choice of alloying metals depends on the intended use of the resulting contact material. The binary Au alloys with typically <10 wt% of other precious metals such as Pt, Pd, or Ag or non-precious metals like Ni, Co, and Cu are the more commonly used ones Table 2 (Tab. 2.2). On one hand these alloy additions improve the mechanical strength and electrical switching properties but on the other hand reduce the electrical conductivity and chemical corrosion resistance Figure 1 (Fig. 2.2) to varying degrees.
Under the aspect of reducing the gold content ternary alloys with a gold content of approximately 70 wt% and additions of Ag and Cu or Ag and Ni resp., for example AuAg25Cu5 or AuAg20Cu10 are used which exhibit for many applications good mechanical stability while at the same time have sufficient resistance against the formation of corrosion layers Table 3 (Table 2.3).
Designation | Composition Au (min. content) | Impurites ppm | Remarks on forms and application |
|---|---|---|---|
Electronic Glod Gold | 99.999 | Cu < 3 Ag < 3 Ca < 1 Mg <1 Fe < 1 | Wires, strips, alloying metal for semiconductors, electronic components |
Pure Gold | 99.995 | Cu < 10 Ag < 15 Ca < 20 Mg < 10 Fe < 3 Si < 10 Pb < 20 | Granulate for high purity alloys, strips, tubing, profiles |
Ingot Grade-Gold | 99.95 | Cu < 100 Ag < 150 Ca < 50 Mg < 50 Fe < 30 Si < 10 | Alloys, commonly used grade |
| Material | Hardness Condition | Tensile Strength Rm [MPa] min. | Elongation A10 [%] min. | Vickers Hardness HV |
|---|---|---|---|---|
| Au | R 140 R 170 R 200 R 240 |
140 170 200 240 |
30 3 2 1 |
20 50 60 70 |
| AuAg20 | R 190 R 250 R 320 R 390 |
190 250 320 390 |
25 2 1 1 |
38 70 95 115 |
| AuAg30 | R 220 R 260 R 320 R 380 |
220 260 320 380 |
25 3 1 1 |
45 75 95 110 |
| AuAg25Cu5 | R 400 R 470 R 570 R 700 |
400 470 570 700 |
25 4 2 2 |
90 120 160 185 |
| AuAg20Cu10 | R 480 R 560 R 720 R 820 |
480 560 720 820 |
20 3 1 1 |
125 145 190 230 |
| AuAg26Ni3 | R 350 R 420 R 500 R 570 |
350 420 500 570 |
20 2 1 1 |
85 110 135 155 |
| AuAg25Pt6 | R 280 R 330 R 410 R 480 |
280 330 410 480 |
18 2 1 1 |
60 90 105 125 |
| AuCo5 | R 340 R 390 R 450 R 530 |
340 390 450 530 |
10 2 1 1 |
95 105 120 150 |
| AuCo5 prec.hardened | heterogeneous | 360 | 3 | 110-130 |
| AuNi5 | R 380 R 450 R 560 R 640 |
380 450 560 640 |
25 3 2 1 |
115 135 160 190 |
| AuPt10 | R 260 R 310 R 370 R 410 |
260 310 370 410 |
20 2 1 1 |
80 90 100 105 |
| AuCu14Pt9Ag4 | R 620 R 700 R 850 R 950 prec.hardened |
620 700 850 950 900 |
20 3 2 1 3 |
190 225 260 270 280 |
Other ternary alloys based on the AuAg system are AuAg26Ni3 and AuAg25Pt6. These alloys are mechanically similar to the AuAgCu alloys but have significantly higher oxidation resistance at elevated temperatures Table 4(Table 2.4).
Material | Properties | ||
|---|---|---|---|
Au | Highest corrosion resistance, low hardness | High electr. conductivity, strong tendency to cold welding | |
AuAg8 | High corrosion resistance, low thermo e.m.f. | Low contact resistance | |
AuPt10 AuPd5 | Very high corrosion resistance | High hardness | |
AuAg10 - 30 | Mostly corrosion resistant | Higher hardness | |
AuNi5 AuCo5 | High corrosion resistance, low tendency to material transfer | High hardness | |
AuAg25Pt6 | High corrosion resistance, low contact resistance | High hardness | |
AuAg26Ni3 AuAg25Cu5 AuAg20Cu10 | Limited corrosion resistance | High hardness | |
AuPd40 AuPd35Ag10 AuCu14Pt9Ag4 | High corrosion resistance | High hardness and mechanical wear resistance | |
Caused by higher gold prices over the past years the development of alloys with further reduced gold content had a high priority. The starting point has been the AuPd system which has continuous solubility of the two components. Besides the binary alloy of AuPd40 and the ternary one AuPd35Ag9 other multiple component alloys were developed. These alloys typically have < 50 wt% Au and often can be solution hardened in order to obtain even higher hardness and tensile strength. They are mostly used in sliding contact applications.
Gold alloys are used in the form of welded wire or profile (also called weldtapes), segments, contact rivets, and stampings produced from clad strip materials. The selection of the bonding process is based on the cost for the joining process, and most importantly on the economical aspect of using the least possible amount of the expensive precious metal component.
Besides being used as switching contacts in relays and pushbuttons, gold alloys are also applied in the design of connectors as well as sliding contacts for potentiometers, sensors, slip rings, and brushes in miniature DC motors Table 5(Table 2.5).
Material | Application Examples | Form of Application |
|---|---|---|
Pure Gold (electroplated) | Corrosion protection layer for contact parts, stationary contacts, bonding surfaces | Electroplated coatings, bond surface layers |
Hard Gold (sputtered) | Contact parts for connectors and switches, sliding contact tracks, bonding surfaces | Electroplated coatings on contact rivets and stamped parts |
Hard Gold (sputtered) | Contacts in switches and relays for low loads, electronic signal relays | Contact surface layer on miniature profiles (weld tapes) |
AuAg8 | Dry circuit switching contacts, electronic signal relays | Contact rivets, welded contact parts |
AuAg20 | Switching contacts for low loads, electronic signal relays | Contact rivets, welded contact parts |
AuAg25Cu5 AuAg25Cu10 AuAg26Ni3 | Contact parts for connectors, switches and relays | Claddings on Cu alloys, contact rivets, contact layer on micro profiles (weld tapes) |
AuNi5 AuCo5 (heterogen) | Contacts in switches and relays for low and medium loads, material transfer resistant contacts | Contact rivets, welded contact parts, contact layer on miniature profiles (weld tapes) |
AuPt10 AuAg25Pt6 | Contacts for highest chemical corrosion resistance in switches and relays | Contact rivets, contact layer on micro profiles (weld tapes) |
AuCu14Pt9Ag4 | Sliding contacts for measurement data transfer | Wire-formed parts |
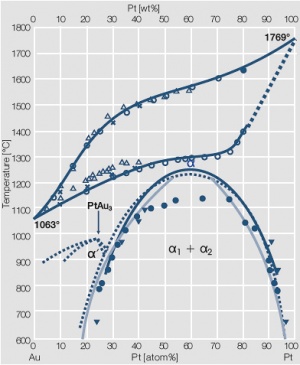
Figure 2 Fig. 2.3: Phase diagram of goldplatinum
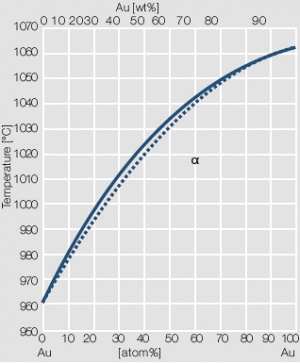
Figure 3 Fig. 2.4: Phase diagram of gold-silver
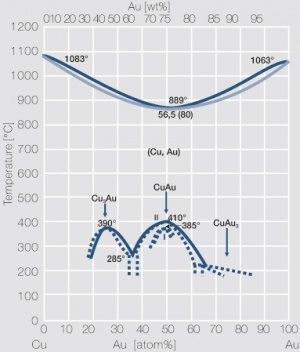
Figure 4 Fig. 2.5: Phase diagram of gold-copper
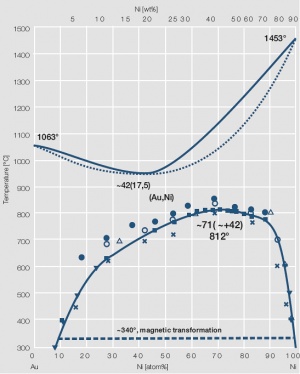
Figure 5 Fig. 2.6: Phase diagram of gold-nickel
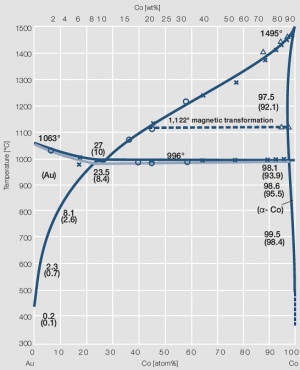
Figure 6 Fig. 2.7: Phase diagram of gold-cobalt
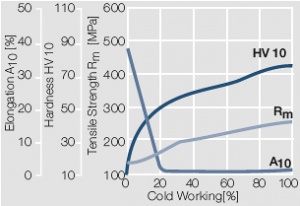
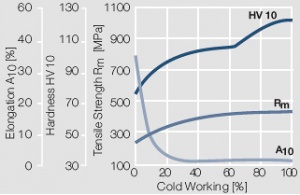
Figure 7 Fig. 2.8: Strain hardening of Au by cold working
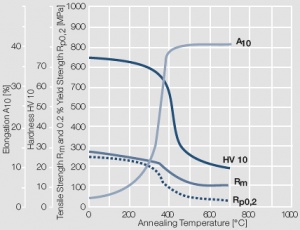
Figure 8 Fig. 2.9: Softening of Au after annealing for 0.5 hrs after 80% cold working
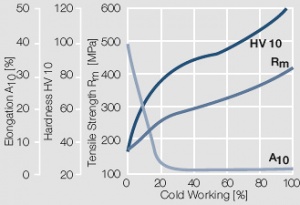
Figure 9 Fig. 2.10: Strain hardening of AuPt10 by cold working
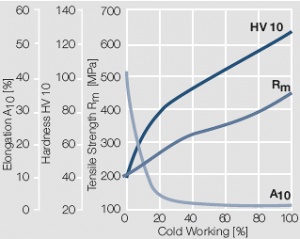
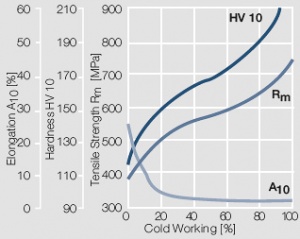
Figure 10 Fig. 2.11: Strain hardening of AuAg20 by cold working
Figure 11 Fig. 2.12: Strain hardening of AuAg30 by cold working
Figure 12 Fig. 2.13: Strain hardening of AuNi5 by cold working
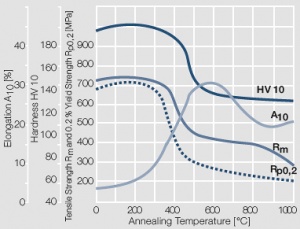
Figure 13 Fig. 2.14: Softening of AuNi5 after annealing for 0.5 hrs after 80% cold working
Figure 14 Fig. 2.15: Strain hardening of AuCo5 by cold working
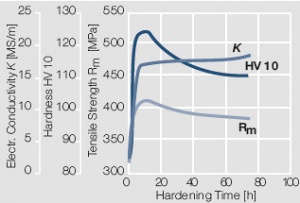
Figure 15 Fig. 2.16: Precipitation hardening of AuCo5 at 400°C hardening temperature
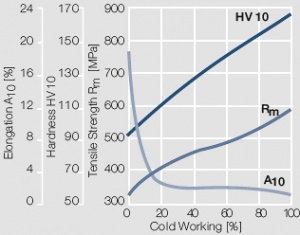
Figure 16 Fig. 2.17: Strain hardening of AuAg25Pt6 by cold working
Figure 17 Fig. 2.18: Strain hardening of AuAg26Ni3 by cold working
Figure 18 Fig. 2.19: Softening of AuAg26Ni3 after annealing for 0.5 hrs after 80% cold working
Figure 19 Fig. 2.20: Strain hardening of AuAg25Cu5 by cold working
Figure 20 Fig. 2.21: Strain hardening of AuAg20Cu10 by cold working
Figure 21 Fig. 2.22: Softening of AuAg20Cu10 after annealing for 0.5 hrs after 80% cold working
Figure 22 Fig. 2.23: Strain hardening of AuCu14Pt9Ag4 by cold working
Figure 23 Fig. 2.24: Precipitation hardening of AuCu14Pt9Ag4 at different hardening temperatures after 50% cold working