|
|
| (24 intermediate revisions by 4 users not shown) |
| Line 1: |
Line 1: |
| − | The following tables list the physical properties of the most technically significant pure metals as well as carbon. The values given may vary considerably, depending on the degree of purity and sometimes they are also difficult to determine. In compiling the data from the available literature, we selected those that are currently the most probable. Some properties are anisotropic and vary with the crystalline structure of the metal.</onlyinclude> In those cases, we listed the value applicable to the poly-crystalline stage. <br> | + | The following tables list the physical properties of the most technically significant pure metals as well as carbon. The values given may vary considerably depending on the degree of purity and sometimes they are also difficult to determine. In compiling the data from the available literature we selected those that are currently the most probable. Some properties are anisotropic and vary with the crystalline structure of the metal.</onlyinclude> In those cases, whenever possible, we listed the value applicable to the poly-crystalline stage. <br> |
| | | | |
| | <figtable id="tab:Mechanical Properties of the Most Important Metals"> | | <figtable id="tab:Mechanical Properties of the Most Important Metals"> |
| − | <caption>'''Mechanical Properties of the Most Important Metals'''</caption>
| + | '''Tab. Mechanical Properties of the Most Important Metals''' |
| | | | |
| | {| class="twocolortable" style="text-align: left; font-size: 12px" | | {| class="twocolortable" style="text-align: left; font-size: 12px" |
| Line 13: |
Line 13: |
| | !Shear Modulus | | !Shear Modulus |
| | [GPa] | | [GPa] |
| − | !Transvers Contraction Coeffic. | + | !Transvers ContractionCoeffic. |
| | |- | | |- |
| | |Aluminum | | |Aluminum |
| Line 226: |
Line 226: |
| | |- | | |- |
| | |} | | |} |
| | + | </figtable> |
| | <div id="text-reference"><sub>1</sub> at 20°C</div> | | <div id="text-reference"><sub>1</sub> at 20°C</div> |
| − | </figtable>
| |
| − | <br/>
| |
| − | <br/>
| |
| − | <figtable id="tab:Atomic properties of the most important metals">
| |
| − | <caption>'''Atomic properties of the most important metals'''</caption>
| |
| − |
| |
| − | {| class="twocolortable" style="text-align: left; font-size: 12px"
| |
| − | |-
| |
| − | !Element/Metal
| |
| − | !Chemical<br/>Symbol
| |
| − | !Atomic Number
| |
| − | !Atomic Weight
| |
| − | !Crystal Structure [[#text-reference1|<sup>1</sup>]]
| |
| − | !Lattic Parameters [[#text-reference1|<sup>1</sup>]]<br />a or b [[#text-reference2|<sup>2</sup>]]<br />[10<sup>10</sup>m]
| |
| − | !Lattic Parameters [[#text-reference1|<sup>1</sup>]]<br />a or b [[#text-reference2|<sup>2</sup>]]<br />[10<sup>-10</sup>m]
| |
| − | !Work Function<br />[eV]
| |
| − | !Ionization Potential<br/>[eV]
| |
| − | |-
| |
| − | |Aluminum
| |
| − | |Al
| |
| − | |13
| |
| − | |26,98
| |
| − | |foc
| |
| − | |4,049
| |
| − | |
| |
| − | |4,08 - 4,3
| |
| − | |5,98
| |
| − | |-
| |
| − | |Antimony
| |
| − | |Sb
| |
| − | |51
| |
| − | |121,75
| |
| − | |rhl
| |
| − | |4,507
| |
| − | |
| |
| − | |4,1
| |
| − | |8,64
| |
| − | |-
| |
| − | |Beryllium
| |
| − | |Be
| |
| − | |4
| |
| − | |9,01
| |
| − | |hcp
| |
| − | |2,286
| |
| − | |3,584
| |
| − | |3,2 - 3,9
| |
| − | |9,32
| |
| − | |-
| |
| − | |Lead
| |
| − | |Pb
| |
| − | |87
| |
| − | |207,19
| |
| − | |fcc
| |
| − | |4,949
| |
| − | |
| |
| − | |4,0 - 4,1
| |
| − | |7,42
| |
| − | |-
| |
| − | |Cadmium
| |
| − | |Cd
| |
| − | |48
| |
| − | |112,40
| |
| − | |hcp
| |
| − | |2,979
| |
| − | |5,617
| |
| − | |3,7 - 4,1
| |
| − | |8,99
| |
| − | |-
| |
| − | |Chromium
| |
| − | |Cr
| |
| − | |24
| |
| − | |52,00
| |
| − | |bcc
| |
| − | |2,884
| |
| − | |
| |
| − | |4,4 - 4,7
| |
| − | |6,76
| |
| − | |-
| |
| − | |Iron
| |
| − | |Fe
| |
| − | |26
| |
| − | |55,85
| |
| − | |bcc
| |
| − | |2,866
| |
| − | |
| |
| − | |4,1 - 4,5
| |
| − | |7,9
| |
| − | |-
| |
| − | |Gallium
| |
| − | |Ga
| |
| − | |31
| |
| − | |69,72
| |
| − | |ort
| |
| − | |4,524
| |
| − | |7,661
| |
| − | |3,8 - 4,1
| |
| − | |6,0
| |
| − | |-
| |
| − | |Gold
| |
| − | |Au
| |
| − | |79
| |
| − | |196,97
| |
| − | |fcc
| |
| − | |4,078
| |
| − | |
| |
| − | |4,3 - 5,1
| |
| − | |9,22
| |
| − | |-
| |
| − | |Indium
| |
| − | |In
| |
| − | |49
| |
| − | |114,82
| |
| − | |tet
| |
| − | |4,594
| |
| − | |4,951
| |
| − | |4,0
| |
| − | |5,79
| |
| − | |-
| |
| − | |Iridium
| |
| − | |Ir
| |
| − | |77
| |
| − | |192,20
| |
| − | |fcc
| |
| − | |3,839
| |
| − | |
| |
| − | |4,6 - 5,3
| |
| − | |9,1
| |
| − | |-
| |
| − | |Cobalt
| |
| − | |Co
| |
| − | |27
| |
| − | |58,93
| |
| − | |hcp
| |
| − | |2,507
| |
| − | |4,069
| |
| − | |4,4 - 4,6
| |
| − | |7,86
| |
| − | |-
| |
| − | |Carbon (Graphite)
| |
| − | |C
| |
| − | |6
| |
| − | |12,01
| |
| − | |hcp-layered lattic[[#text-reference3|<sup>3</sup>]]
| |
| − | |2,456
| |
| − | |6,696
| |
| − | |4,8
| |
| − | |11,27
| |
| − | |-
| |
| − | |Copper
| |
| − | |Cu
| |
| − | |29
| |
| − | |63,54
| |
| − | |fcc
| |
| − | |3,615
| |
| − | |
| |
| − | |4,4
| |
| − | |7,72
| |
| − | |-
| |
| − | |Magnesium
| |
| − | |Mg
| |
| − | |12
| |
| − | |24,31
| |
| − | |hcp
| |
| − | |3,209
| |
| − | |5,210
| |
| − | |3,7
| |
| − | |7,64
| |
| − | |-
| |
| − | |Manganese
| |
| − | |Mn
| |
| − | |25
| |
| − | |54,94
| |
| − | |complex cubic
| |
| − | |8,912
| |
| − | |
| |
| − | |3,8 - 4,1
| |
| − | |6,0
| |
| − | |-
| |
| − | |Molybdenum
| |
| − | |Mo
| |
| − | |42
| |
| − | |95,94
| |
| − | |bcc
| |
| − | |3,147
| |
| − | |
| |
| − | |4,1 - 4,5
| |
| − | |7,18
| |
| − | |-
| |
| − | |Nickel
| |
| − | |Ni
| |
| − | |28
| |
| − | |58,71
| |
| − | |fcc
| |
| − | |3,524
| |
| − | |
| |
| − | |5,0 - 5,2
| |
| − | |7,63
| |
| − | |-
| |
| − | |Niobium
| |
| − | |Nb
| |
| − | |41
| |
| − | |92,91
| |
| − | |bcc
| |
| − | |3,301
| |
| − | |
| |
| − | |4,0
| |
| − | |6,77
| |
| − | |-
| |
| − | |Osmium
| |
| − | |Os
| |
| − | |76
| |
| − | |190,23
| |
| − | |hcp
| |
| − | |2,734
| |
| − | |4,320
| |
| − | |4,5
| |
| − | |8,7
| |
| − | |-
| |
| − | |Palladium
| |
| − | |Pd
| |
| − | |46
| |
| − | |106,40
| |
| − | |fcc
| |
| − | |3,890
| |
| − | |
| |
| − | |4,5 - 5,0
| |
| − | |8,34
| |
| − | |-
| |
| − | |Platinum
| |
| − | |Pt
| |
| − | |78
| |
| − | |195,09
| |
| − | |fcc
| |
| − | |3,931
| |
| − | |
| |
| − | |4,1 - 5,5
| |
| − | |9,0
| |
| − | |-
| |
| − | |Mercury
| |
| − | |Hg
| |
| − | |80
| |
| − | |200,59
| |
| − | |rhl[[#text-reference4|<sup>4</sup>]]
| |
| − | |3,061[[#text-reference4|<sup>4</sup>]]
| |
| − | |
| |
| − | |4,5
| |
| − | |10,44
| |
| − | |-
| |
| − | |Rhenium
| |
| − | |Re
| |
| − | |75
| |
| − | |186,20
| |
| − | |hcp
| |
| − | |2,760
| |
| − | |4,458
| |
| − | |4,7 - 5,0
| |
| − | |7,8
| |
| − | |-
| |
| − | |Rhodium
| |
| − | |Rh
| |
| − | |45
| |
| − | |102,91
| |
| − | |fcc
| |
| − | |3,804
| |
| − | |
| |
| − | |4,6 - 4,9
| |
| − | |7,46
| |
| − | |-
| |
| − | |Ruthenium
| |
| − | |Ru
| |
| − | |44
| |
| − | |101,07
| |
| − | |hcp
| |
| − | |2,704
| |
| − | |4,281
| |
| − | |4,5
| |
| − | |7,37
| |
| − | |-
| |
| − | |Silver
| |
| − | |Ag
| |
| − | |47
| |
| − | |107,87
| |
| − | |fcc
| |
| − | |4,086
| |
| − | |
| |
| − | |4,3
| |
| − | |7,57
| |
| − | |-
| |
| − | |Tantalum
| |
| − | |Ta
| |
| − | |73
| |
| − | |180,95
| |
| − | |bcc
| |
| − | |3,303
| |
| − | |
| |
| − | |4,0 - 4,2
| |
| − | |7,89
| |
| − | |-
| |
| − | |Titanium
| |
| − | |Ti
| |
| − | |2
| |
| − | |47,90
| |
| − | |hcp
| |
| − | |2,950
| |
| − | |4,683
| |
| − | |4,0 - 4,4
| |
| − | |6,83
| |
| − | |-
| |
| − | |Vanadium
| |
| − | |V
| |
| − | |23
| |
| − | |50,94
| |
| − | |bcc
| |
| − | |3,039
| |
| − | |
| |
| − | |3,8 - 4,2
| |
| − | |6,71
| |
| − | |-
| |
| − | |Bismuth
| |
| − | |Bi
| |
| − | |83
| |
| − | |208,98
| |
| − | |rhl
| |
| − | |4,746
| |
| − | |
| |
| − | |4,1 - 4,5
| |
| − | |8,0
| |
| − | |-
| |
| − | |Tungsten
| |
| − | |W
| |
| − | |74
| |
| − | |183,85
| |
| − | |bcc
| |
| − | |3,158
| |
| − | |
| |
| − | |4,3 - 5,0
| |
| − | |7,98
| |
| − | |-
| |
| − | |Zinc
| |
| − | |Zn
| |
| − | |30
| |
| − | |65,37
| |
| − | |hcp
| |
| − | |2,665
| |
| − | |4,947
| |
| − | |3,1 - 4,3
| |
| − | |9,39
| |
| − | |-
| |
| − | |Tin
| |
| − | |Sn
| |
| − | |50
| |
| − | |118,69
| |
| − | |tet
| |
| − | |5,831
| |
| − | |3,181
| |
| − | |3,6 - 4,1
| |
| − | |7,33
| |
| − | |-
| |
| − | |Zirconium
| |
| − | |Zr
| |
| − | |40
| |
| − | |91,22
| |
| − | |hcp
| |
| − | |3,231
| |
| − | |5,148
| |
| − | |3,7 - 4,3
| |
| − | |6,92
| |
| − | |-
| |
| − | |}
| |
| − | <div id="text-reference1"><sub>1</sub> at 20°C</div>
| |
| − | <div id="text-reference2"><sub>2</sub> for rhombohedral crystals, the rhombohedra angle α is given in angle degrees and minutes; for orthorhombic crystals the parameter β is shown in m x 10<sup>-10</sup></div>
| |
| − | <div id="text-reference3"><sub>3</sub> α-crystal</div>
| |
| − | <div id="text-reference4"><sub>4</sub> at -50°C</div>
| |
| − | </figtable>
| |
| − | fcc = cubic face cenered // bcc = cubic body centered // hcp = hexagonal dense spherical
| |
| − | ort = orthorhombic // tet = tetragonal // rhl = rhombohedral
| |
| − | <br/>
| |
| − | <br/>
| |
| − | <figtable id="tab:Thermal properties of the most important metals">
| |
| − | <caption>'''Thermal properties of the most important metals'''</caption>
| |
| | | | |
| − | {| class="twocolortable" style="text-align: left; font-size: 12px"
| + | '''Tab. Atomic Properties of the Most Important Metals''' |
| − | |-
| |
| − | !Element/Metal
| |
| − | !Specific Heat [[#text-reference5|<sup>1</sup>]]<br/>[kJ/(K*kg)]
| |
| − | !Softening<br/>Temperature<br/>[°C]
| |
| − | !Melting Point<br/>[°C]
| |
| − | !Heat of Fusion<br/>[kJ/kg]
| |
| − | !Vapor Pressure<br/>at Melting Point<br/>[Pa]
| |
| − | !Boiling Point<br/>[°C]
| |
| − | !Heat of Vaporizing<br />[kJ/g]
| |
| − | !Thermal<br/>Conductivity<br/>[W/(m*K)]
| |
| − | !Linear Expansion<br/>Coefficient[[#text-reference6|<sup>2</sup>]]<br />[10<sup>-6</sup>m/K]
| |
| − | !Volume Change at<br/>Solidification<br/>[%]
| |
| − | |-
| |
| − | |Aluminum
| |
| − | |0,900
| |
| − | |150
| |
| − | |660
| |
| − | |398
| |
| − | |2,5x10<sup>-6</sup>
| |
| − | |2467
| |
| − | |10,47
| |
| − | |237
| |
| − | |23,6
| |
| − | | -6,5
| |
| − | |-
| |
| − | |Antimony
| |
| − | |0,210
| |
| − | |
| |
| − | |630
| |
| − | |163
| |
| − | |2,5x10<sup>-9</sup>
| |
| − | |1587
| |
| − | |1,97
| |
| − | |24,3
| |
| − | |10,5
| |
| − | | +9,5
| |
| − | |-
| |
| − | |Beryllium
| |
| − | |1,824
| |
| − | |
| |
| − | |1277
| |
| − | |1090
| |
| − | |4,3
| |
| − | |2477
| |
| − | |
| |
| − | |200
| |
| − | |12,3
| |
| − | |
| |
| − | |-
| |
| − | |Lead
| |
| − | |0,130
| |
| − | |200
| |
| − | |327
| |
| − | |25
| |
| − | |4,21x10<sup>-7</sup>
| |
| − | |1750
| |
| − | |24,70
| |
| − | |35,3
| |
| − | |29,3
| |
| − | | -3,5
| |
| − | |-
| |
| − | |Cadmium
| |
| − | |0,230
| |
| − | |
| |
| − | |321
| |
| − | |54
| |
| − | |14,8
| |
| − | |767
| |
| − | |0,88
| |
| − | |96,8
| |
| − | |41,0
| |
| − | | -4,0
| |
| − | |-
| |
| − | |Chromium
| |
| − | |0,450
| |
| − | |
| |
| − | |1857
| |
| − | |314
| |
| − | |990
| |
| − | |2672
| |
| − | |5,86
| |
| − | |93,7
| |
| − | |6,2
| |
| − | |
| |
| − | |-
| |
| − | |Iron
| |
| − | |0,444
| |
| − | |500
| |
| − | |1537
| |
| − | |268
| |
| − | |7,05
| |
| − | |2750
| |
| − | |80,2
| |
| − | |12,2
| |
| − | | -3,0
| |
| − | |
| |
| − | |-
| |
| − | |Gallium
| |
| − | |0,370
| |
| − | |
| |
| − | |29,8
| |
| − | |80,4
| |
| − | |9,6x10<sup>-36</sup>
| |
| − | |2204
| |
| − | |3,90
| |
| − | |40,6
| |
| − | |18,0
| |
| − | | +3,0
| |
| − | |-
| |
| − | |Gold
| |
| − | |0,128
| |
| − | |100
| |
| − | |1064
| |
| − | |63
| |
| − | |2,4x10<sup>-3</sup>
| |
| − | |3080
| |
| − | |1,55
| |
| − | |317
| |
| − | |14,3
| |
| − | | -5,1
| |
| − | |-
| |
| − | |Indium
| |
| − | |0,233
| |
| − | |
| |
| − | |157
| |
| − | |28,5
| |
| − | |1,5x10<sup>-17</sup>
| |
| − | |2072
| |
| − | |1,97
| |
| − | |81,6
| |
| − | |
| |
| − | | -2,5
| |
| − | |-
| |
| − | |Iridium
| |
| − | |0,130
| |
| − | |
| |
| − | |2410
| |
| − | |144
| |
| − | |1,5
| |
| − | |4130
| |
| − | |3,31
| |
| − | |147
| |
| − | |6,5
| |
| − | |
| |
| − | |-
| |
| − | |Cobalt
| |
| − | |0,420
| |
| − | |
| |
| − | |1495
| |
| − | |260
| |
| − | |175
| |
| − | |2927
| |
| − | |6,66
| |
| − | |100
| |
| − | |13,8
| |
| − | |
| |
| − | |-
| |
| − | |Carbon (Graphite)
| |
| − | |0,720
| |
| − | |
| |
| − | |
| |
| − | |
| |
| − | |
| |
| − | |3825 sublimiert
| |
| − | |119 - 165
| |
| − | |155
| |
| − | |
| |
| − | |
| |
| − | |-
| |
| − | |Copper
| |
| − | |0,385
| |
| − | |190
| |
| − | |1084
| |
| − | |205
| |
| − | |5,2x10<sup>-2</sup>
| |
| − | |2567
| |
| − | |4,77
| |
| − | |401
| |
| − | |16,5
| |
| − | | -4,2
| |
| − | |-
| |
| − | |Magnesium
| |
| − | |1,020
| |
| − | |
| |
| − | |650
| |
| − | |373
| |
| − | |361
| |
| − | |1107
| |
| − | |5,44
| |
| − | |156
| |
| − | |26,0
| |
| − | | -4,1
| |
| − | |-
| |
| − | |Manganese
| |
| − | |0,480
| |
| − | |
| |
| − | |1244
| |
| − | |264
| |
| − | |121
| |
| − | |1962
| |
| − | |4,10
| |
| − | |7,8
| |
| − | |23,0
| |
| − | | -1,7
| |
| − | |-
| |
| − | |Molybdenum
| |
| − | |0,250
| |
| − | |900
| |
| − | |2623
| |
| − | |292
| |
| − | |3,6
| |
| − | |4639
| |
| − | |5,61
| |
| − | |138
| |
| − | |5,2
| |
| − | |
| |
| − | |-
| |
| − | |Nickel
| |
| − | |0,440
| |
| − | |520
| |
| − | |1453
| |
| − | |301
| |
| − | |237
| |
| − | |2913
| |
| − | |6,45
| |
| − | |90,7
| |
| − | |13,0
| |
| − | | -2,5
| |
| − | |-
| |
| − | |Niobium
| |
| − | |0,272
| |
| − | |
| |
| − | |2477
| |
| − | |289
| |
| − | |7,9x10<sup>-2</sup>
| |
| − | |4744
| |
| − | |7,79
| |
| − | |53,7
| |
| − | |7,3
| |
| − | |
| |
| − | |-
| |
| − | |Osmium
| |
| − | |0,130
| |
| − | |
| |
| − | |3045
| |
| − | |141
| |
| − | |2,52
| |
| − | |5012
| |
| − | |3,81
| |
| − | |87,6
| |
| − | |6,5
| |
| − | |
| |
| − | |-
| |
| − | |Palladium
| |
| − | |0,244
| |
| − | |
| |
| − | |1554
| |
| − | |143
| |
| − | |1,33
| |
| − | |2970
| |
| − | |3,48
| |
| − | |71,8
| |
| − | |11,1
| |
| − | | -5,5
| |
| − | |-
| |
| − | |Platinum
| |
| − | |0,130
| |
| − | |540
| |
| − | |1772
| |
| − | |113
| |
| − | |3,2x10<sup>-2</sup>
| |
| − | |3827
| |
| − | |2,62
| |
| − | |71,6
| |
| − | |9,0
| |
| − | | -6,0
| |
| − | |-
| |
| − | |Mercury
| |
| − | |0,140
| |
| − | |
| |
| − | | -38,9
| |
| − | |11,7
| |
| − | |3,1x10<sup>-4</sup>
| |
| − | |357
| |
| − | |0,29
| |
| − | |8,34
| |
| − | |60,8
| |
| − | | -3,7
| |
| − | |-
| |
| − | |Rhenium
| |
| − | |0,137
| |
| − | |
| |
| − | |3186
| |
| − | |178
| |
| − | |3,24
| |
| − | |5596
| |
| − | |3,42
| |
| − | |72
| |
| − | |6,7
| |
| − | |
| |
| − | |-
| |
| − | |Rhodium
| |
| − | |0,242
| |
| − | |
| |
| − | |1966
| |
| − | |211
| |
| − | |6,36x10<sup>-1</sup>
| |
| − | |3695
| |
| − | |5,19
| |
| − | |150
| |
| − | |8,5
| |
| − | | -10,8
| |
| − | |-
| |
| − | |Ruthenium
| |
| − | |0,238
| |
| − | |
| |
| − | |2310
| |
| − | |252
| |
| − | |1,4
| |
| − | |4150
| |
| − | |6,62
| |
| − | |117
| |
| − | |9,5
| |
| − | |
| |
| − | |-
| |
| − | |Silver
| |
| − | |0,232
| |
| − | |180
| |
| − | |961,9
| |
| − | |105
| |
| − | |3,4x10<sup>-1</sup>
| |
| − | |2212
| |
| − | |2,39
| |
| − | |429
| |
| − | |19,5
| |
| − | | -3,8
| |
| − | |-
| |
| − | |Tantalum
| |
| − | |0,140
| |
| − | |850
| |
| − | |3017
| |
| − | |157
| |
| − | |7,86x10<sup>-1</sup>
| |
| − | |5448
| |
| − | |4,32
| |
| − | |57,5
| |
| − | |6,5
| |
| − | |
| |
| − | |-
| |
| − | |Titanium
| |
| − | |0,520
| |
| − | |
| |
| − | |1668
| |
| − | |403
| |
| − | |4,9x10<sup>-1</sup>
| |
| − | |2830
| |
| − | |8,80
| |
| − | |21,9
| |
| − | |10,8
| |
| − | |
| |
| − | |-
| |
| − | |Vanadium
| |
| − | |0,490
| |
| − | |
| |
| − | |1902
| |
| − | |330
| |
| − | |3,06
| |
| − | |3287
| |
| − | |10,3
| |
| − | |30,7
| |
| − | |8,3
| |
| − | |
| |
| − | |-
| |
| − | |Bismuth
| |
| − | |0,122
| |
| − | |
| |
| − | |271
| |
| − | |54
| |
| − | |6,5x10<sup>-4</sup>
| |
| − | |1564
| |
| − | |1,43
| |
| − | |7,87
| |
| − | |14,0
| |
| − | | -0,33
| |
| − | |-
| |
| − | |Tungsten
| |
| − | |0,138
| |
| − | |1000
| |
| − | |3422
| |
| − | |193
| |
| − | |4,27
| |
| − | |5555
| |
| − | |3,98
| |
| − | |174
| |
| − | |4,5
| |
| − | |
| |
| − | |-
| |
| − | |Zinc
| |
| − | |0,385
| |
| − | |170
| |
| − | |420
| |
| − | |100
| |
| − | |3,06
| |
| − | |907
| |
| − | |1,76
| |
| − | |116
| |
| − | |36,0
| |
| − | | -4,7
| |
| − | |-
| |
| − | |Tin
| |
| − | |0,228
| |
| − | |100
| |
| − | |222
| |
| − | |59
| |
| − | |6x10<sup>-21</sup>
| |
| − | |2602
| |
| − | |1,95
| |
| − | |66,6
| |
| − | |26,7
| |
| − | | -2,8
| |
| − | |-
| |
| − | |Zirconium
| |
| − | |0,281
| |
| − | |
| |
| − | |1852
| |
| − | |224
| |
| − | |1,7x10<sup>-3</sup>
| |
| − | |4409
| |
| − | |4,6
| |
| − | |22,7
| |
| − | |5,9
| |
| − | |
| |
| − | |-
| |
| − | |}
| |
| − | <div id="text-reference5"><sub>1</sub> at 20°C</div>
| |
| − | <div id="text-reference6"><sub>2</sub> between 20°C and 100°C</div>
| |
| − | </figtable>
| |
| − | <br/>
| |
| − | <br/>
| |
| − | <figtable id="tab:Electrical Properties of the Most Important Metals">
| |
| − | <caption>'''Electrical Properties of the Most Important Metals'''</caption>
| |
| | | | |
| − | {| class="twocolortable" style="text-align: left; font-size: 12px"
| + | '''Tab. Thermal Properties of the Most Important Metals''' |
| − | |-
| |
| − | !Element/Metal
| |
| − | !Electrical Resistivity [[#text-reference7|<sup>1</sup>]]<br/>[Ω*mm<sup>2</sup>/m]
| |
| − | !Electrical Conductivity[[#text-reference7|<sup>1</sup>]]<br/>[MS/m]
| |
| − | !Temperatur Coeff.<br/>of Electrical<br/>Resistance[[#text-reference8|<sup>2</sup>]]<br/>[10<sup>-3</sup>/K]
| |
| − | !Absolute thermal<br/>e.m.f.[[#text-reference9|<sup>3</sup>]]<br/>[µV/K]
| |
| − | !Critical<br/>Superconductor<br/>Temperatur<br/>[K]
| |
| − | !Softening<br/>Voltage<br/>(measured)<br/>[V]
| |
| − | !Melting<br/>Voltage<br/>(measured)<br/>[V]
| |
| − | !Melting<br/>Voltage<br/>(calculated)[[#text-reference10|<sup>4</sup>]]<br/>[V]
| |
| − | !Minimum Arc<br/>Voltage<br/>[V]
| |
| − | !Minimum Arc<br/>Current<br/>[A]
| |
| − | |-
| |
| − | |Aluminum
| |
| − | |2,65
| |
| − | |37,7
| |
| − | |4,6
| |
| − | | -1,6
| |
| − | |1,18
| |
| − | |0,1
| |
| − | |0,3
| |
| − | |0,29
| |
| − | |11,2
| |
| − | |0,4
| |
| − | |-
| |
| − | |Antimony
| |
| − | |38,6
| |
| − | |2,6
| |
| − | |5,4
| |
| − | | +20,6 - +46,8
| |
| − | |
| |
| − | |0,2
| |
| − | |
| |
| − | |0,28
| |
| − | |10,5
| |
| − | |
| |
| − | |-
| |
| − | |Beryllium
| |
| − | |4,2
| |
| − | |23,8
| |
| − | |10,0
| |
| − | | -3,3
| |
| − | |0,026
| |
| − | |
| |
| − | |
| |
| − | |0,48
| |
| − | |
| |
| − | |
| |
| − | |-
| |
| − | |Lead
| |
| − | |19,2
| |
| − | |5,2
| |
| − | |4,2
| |
| − | | -1,2
| |
| − | |7,196
| |
| − | |
| |
| − | |0,19
| |
| − | |0,17
| |
| − | |11,5
| |
| − | |0,1
| |
| − | |-
| |
| − | |Cadmium
| |
| − | |7,50
| |
| − | |13,30
| |
| − | |4,3
| |
| − | | -0,1 - +3,6
| |
| − | |0,52
| |
| − | |0,12
| |
| − | |
| |
| − | |0,17
| |
| − | |12
| |
| − | |0,4
| |
| − | |-
| |
| − | |Chromium
| |
| − | |14,95
| |
| − | |6,7
| |
| − | |3,0
| |
| − | | +14,0
| |
| − | |3,0
| |
| − | |
| |
| − | |
| |
| − | |0,67
| |
| − | |16
| |
| − | |0,45
| |
| − | |-
| |
| − | |Iron
| |
| − | |9,72
| |
| − | |10,3
| |
| − | |6,6
| |
| − | | +16,0
| |
| − | |
| |
| − | |0,19
| |
| − | |0,6
| |
| − | |0,54
| |
| − | |11,5
| |
| − | |
| |
| − | |-
| |
| − | |Gallium
| |
| − | |43,2
| |
| − | |2,3
| |
| − | |4,0
| |
| − | |
| |
| − | |1,08
| |
| − | |
| |
| − | |
| |
| − | |0,04
| |
| − | |
| |
| − | |0,35
| |
| − | |-
| |
| − | |Gold
| |
| − | |2,35
| |
| − | |42,6
| |
| − | |4,0
| |
| − | | +1,7
| |
| − | |
| |
| − | |0,08
| |
| − | |0,43
| |
| − | |0,42
| |
| − | |15
| |
| − | |
| |
| − | |-
| |
| − | |Indium
| |
| − | |8,37
| |
| − | |11,94
| |
| − | |4,9
| |
| − | |
| |
| − | |3,41
| |
| − | |
| |
| − | |
| |
| − | |0,11
| |
| − | |
| |
| − | |
| |
| − | |-
| |
| − | |Iridium
| |
| − | |5,31
| |
| − | |18,83
| |
| − | |4,1
| |
| − | | +1,5
| |
| − | |0,11
| |
| − | |
| |
| − | |
| |
| − | |0,86
| |
| − | |11,5
| |
| − | |
| |
| − | |-
| |
| − | |Cobalt
| |
| − | |6,24
| |
| − | |16,0
| |
| − | |6,6
| |
| − | | -18,5
| |
| − | |
| |
| − | |
| |
| − | |
| |
| − | |0,54
| |
| − | |
| |
| − | |0,01 - 0,02
| |
| − | |-
| |
| − | |Carbon (Graphite)
| |
| − | |30,0
| |
| − | |3,33
| |
| − | |
| |
| − | |
| |
| − | |
| |
| − | |
| |
| − | |
| |
| − | |
| |
| − | |20
| |
| − | |0,4
| |
| − | |-
| |
| − | |Copper
| |
| − | |1,67
| |
| − | |59,9
| |
| − | |4,3
| |
| − | | +1,7
| |
| − | |
| |
| − | |0,12
| |
| − | |0,43
| |
| − | |0,42
| |
| − | |12 - 13
| |
| − | |
| |
| − | |-
| |
| − | |Magnesium
| |
| − | |4,42
| |
| − | |22,62
| |
| − | |4,2
| |
| − | | +3,4
| |
| − | |
| |
| − | |
| |
| − | |
| |
| − | |0,28
| |
| − | |
| |
| − | |
| |
| − | |-
| |
| − | |Manganese
| |
| − | |185,0
| |
| − | |0,54
| |
| − | |0,5
| |
| − | |
| |
| − | |
| |
| − | |
| |
| − | |
| |
| − | |0,47
| |
| − | |
| |
| − | |0,75
| |
| − | |-
| |
| − | |Molybdenum
| |
| − | |5,20
| |
| − | |19,2
| |
| − | |4,7
| |
| − | | +5,9
| |
| − | |0,92
| |
| − | |0,3
| |
| − | |0,75
| |
| − | |0,91
| |
| − | |12
| |
| − | |0,4 -0,5
| |
| − | |-
| |
| − | |Nickel
| |
| − | |6,85
| |
| − | |14,6
| |
| − | |6,8
| |
| − | | -18,9
| |
| − | |
| |
| − | |0,16
| |
| − | |0,65
| |
| − | |0,54
| |
| − | |14
| |
| − | |
| |
| − | |-
| |
| − | |Niobium
| |
| − | |13,1
| |
| − | |7,6
| |
| − | |3,4
| |
| − | | -0,5
| |
| − | |9,2
| |
| − | |
| |
| − | |
| |
| − | |0,778
| |
| − | |
| |
| − | |
| |
| − | |-
| |
| − | |Osmium
| |
| − | |8,12
| |
| − | |12,31
| |
| − | |4,2
| |
| − | |
| |
| − | |0,66
| |
| − | |
| |
| − | |
| |
| − | |1,04
| |
| − | |
| |
| − | |0,8 -0,9
| |
| − | |-
| |
| − | |Palladium
| |
| − | |10,82
| |
| − | |9,24
| |
| − | |3,8
| |
| − | | -0,9
| |
| − | |3,3
| |
| − | |
| |
| − | |0,57
| |
| − | |0,57
| |
| − | |15 - 16
| |
| − | |0,8 -1,0
| |
| − | |-
| |
| − | |Platinum
| |
| − | |10,54
| |
| − | |9,58
| |
| − | |3,9
| |
| − | | -4,4
| |
| − | |0,0019
| |
| − | |0,25
| |
| − | |0,71
| |
| − | |0,64
| |
| − | |17
| |
| − | |
| |
| − | |-
| |
| − | |Mercury
| |
| − | |94,9
| |
| − | |1,14
| |
| − | |1,0
| |
| − | | +8,5
| |
| − | |4,15
| |
| − | |
| |
| − | |
| |
| − | |
| |
| − | |
| |
| − | |0,35
| |
| − | |-
| |
| − | |Rhenium
| |
| − | |19,3
| |
| − | |5,2
| |
| − | |4,6
| |
| − | |
| |
| − | |1,7
| |
| − | |
| |
| − | |
| |
| − | |1,09
| |
| − | |
| |
| − | |
| |
| − | |-
| |
| − | |Rhodium
| |
| − | |4,51
| |
| − | |22,2
| |
| − | |4,4
| |
| − | | +1,7
| |
| − | |0,000325
| |
| − | |
| |
| − | |
| |
| − | |0,70
| |
| − | |14
| |
| − | |
| |
| − | |-
| |
| − | |Ruthenium
| |
| − | |7,62
| |
| − | |13,12
| |
| − | |4,6
| |
| − | | -18,0
| |
| − | |0,49
| |
| − | |
| |
| − | |
| |
| − | |0,81
| |
| − | |
| |
| − | |0,4
| |
| − | |-
| |
| − | |Silver
| |
| − | |1,59
| |
| − | |62,9
| |
| − | |4,3
| |
| − | | +1,4
| |
| − | |
| |
| − | |0,09
| |
| − | |0,37
| |
| − | |0,38
| |
| − | |12
| |
| − | |
| |
| − | |-
| |
| − | |Tantalum
| |
| − | |12,4
| |
| − | |8,1
| |
| − | |3,5
| |
| − | | -2,3
| |
| − | |4,47
| |
| − | |0,3
| |
| − | |
| |
| − | |1,03
| |
| − | |12
| |
| − | |
| |
| − | |-
| |
| − | |Titanium
| |
| − | |43,5
| |
| − | |2,3
| |
| − | |5,5
| |
| − | | +7,3
| |
| − | |0,4
| |
| − | |
| |
| − | |
| |
| − | |0,61
| |
| − | |
| |
| − | |
| |
| − | |-
| |
| − | |Vanadium
| |
| − | |26,0
| |
| − | |3,8
| |
| − | |3,9
| |
| − | | +1,0
| |
| − | |5,3
| |
| − | |
| |
| − | |
| |
| − | |0,68
| |
| − | |
| |
| − | |
| |
| − | |-
| |
| − | |Bismuth
| |
| − | |12,1
| |
| − | |8,36
| |
| − | |4,5
| |
| − | | -53 - -110
| |
| − | |
| |
| − | |
| |
| − | |
| |
| − | |0,15
| |
| − | |
| |
| − | |
| |
| − | |-
| |
| − | |Tungsten
| |
| − | |5,65
| |
| − | |17,7
| |
| − | |4,8
| |
| − | | +0,8
| |
| − | |0,0154
| |
| − | |1,1
| |
| − | |1,16
| |
| − | |1,16
| |
| − | |
| |
| − | |0,8 - 1,2
| |
| − | |-
| |
| − | |Zinc
| |
| − | |5,92
| |
| − | |16,9
| |
| − | |4,2
| |
| − | | +0,4 - +2,3
| |
| − | |0,85
| |
| − | |0,17
| |
| − | |0,2
| |
| − | |0,20
| |
| − | |15 - 16
| |
| − | |0,1
| |
| − | |-
| |
| − | |Tin
| |
| − | |11,0
| |
| − | |9,09
| |
| − | |4,6
| |
| − | | -0,6 - -1,5
| |
| − | |3,72
| |
| − | |0,13
| |
| − | |0,14
| |
| − | |0,14
| |
| − | |11
| |
| − | |
| |
| − | |-
| |
| − | |Zirconium
| |
| − | |43,5
| |
| − | |2,3
| |
| − | |4,4
| |
| − | | +9,5
| |
| − | |0,55
| |
| − | |
| |
| − | |0,67
| |
| − | |0,67
| |
| − | |12,5
| |
| − | |
| |
| − | |-
| |
| − | |}
| |
| − | <div id="text-reference7"><sub>1</sub> at 20°C</div>
| |
| − | <div id="text-reference8"><sub>2</sub> near room temperature</div>
| |
| − | <div id="text-reference9"><sub>3</sub> near room temperature, however values for metals with non-cubic structure can vary widely</div>
| |
| − | <div id="text-reference10"><sub>4</sub> Calculated according to U<sub>Melt</sub> = [4L * (T<sup>2</sup><sub>Melt</sub> - T<sup>2</sup><sub>0</sub>)]<sup>1/2</sup> mit
| |
| − | U<sub>Melt</sub>= Melting Voltage, L = Lorenz Constant (2,45x10<sup>-8</sup>[V/K], T<sub>Melt</sub>= Melting Temperature, T<sub>0</sub>= Temperature at a point distant from the constriction spot</div>
| |
| − | </figtable>
| |
| | | | |
| | + | '''Tab. Electrical Properties of the Most Important Metals''' |
| | | | |
| | + | <div class="multiple-images"> |
| | + | [[File:Mechanical-Properties-of-the-Most-Important-Metals.jpg|left|thumb|Mechanical Properties of the Most Important Metals]] |
| | + | [[File:Atomic-Properties-of-the-Most-Important-Metals.jpg|left|thumb|Atomic Properties of the Most Important Metals]] |
| | + | [[File:Thermal-Properties-of-the-Most-Important-Metals.jpg|left|thumb|Thermal Properties of the Most Important Metals]] |
| | + | [[File:Electrical-Properties-of-the-Most-Important-Metals.jpg|left|thumb|Electrical Properties of the Most Important Metals]] |
| | + | </div> |
| | + | <div class="clear"></div> |
| | ===References=== | | ===References=== |
| | | | |
| Line 1,520: |
Line 268: |
| | Wyckoff, R., W., G.: Crystal Structures. Vol 1,New York, 1963 | | Wyckoff, R., W., G.: Crystal Structures. Vol 1,New York, 1963 |
| | | | |
| − | [[de:Physikalische_Eigenschaften_der_wichtigsten_Metalle]] | + | [[Category:Metal Powders|Category]] |
| | + | [[Category:Thermal conductivity|Category]] |
The following tables list the physical properties of the most technically significant pure metals as well as carbon. The values given may vary considerably depending on the degree of purity and sometimes they are also difficult to determine. In compiling the data from the available literature we selected those that are currently the most probable. Some properties are anisotropic and vary with the crystalline structure of the metal. In those cases, whenever possible, we listed the value applicable to the poly-crystalline stage.
1 at 20°C
Tab. Atomic Properties of the Most Important Metals
Tab. Thermal Properties of the Most Important Metals
Tab. Electrical Properties of the Most Important Metals
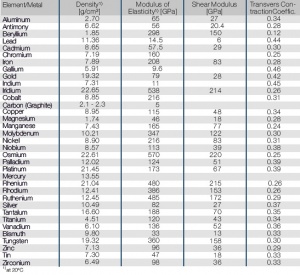
Mechanical Properties of the Most Important Metals
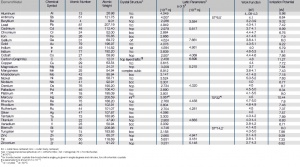
Atomic Properties of the Most Important Metals
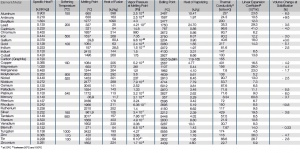
Thermal Properties of the Most Important Metals
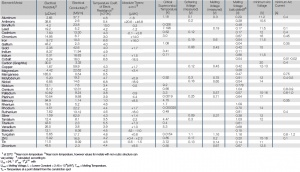
Electrical Properties of the Most Important Metals
References
Metals Handbook, Desk Edition: Chicago, IL, American Society of Metal, 1985
Landolt-Börnstein: Zahlenwerte und Funktionen. Springer-Verlag, Berlin-Göttingen-Heidelberg, 1959
Handbook of Chemistry and Physics, 70th Edition: CRC Press., Inc. Boca Raton, Florida, 1989 - 1990
Fluck, E.; Heumann, K., G.: Periodensystem der Elemente. Weinheim: VCH-Verlagsgesellschaft, 1986
Kieffer, R.; Jangg, G.; Ettmayer, P.: Sondermetalle. Springer- Verlag, Wien-New York, 1963
Hering, E.; Schulz, W.: Physik für Ingenieure (Periodensystem der Elemente). Düsseldorf: VDI-Verlag, 1988
Degussa AG (Hrsg.): Edelmetall-Taschenbuch. Hüthig-Verlag, Heidelberg, 1995
Slade, P.; G. (editor): Electrical Contacts Principles and Applications. Marcel Dekker, Inc., New York-Basel, 1999
Gerritsen, A.; N.: Metallic Conductivity in: Flügge, S.: Handbuch der Physik, Bd. 19, Springer-Verlag, Berlin-Göttingen-Heidelberg, 1956
Köster, W.; Franz, H.: Poisson,s Ratio for Metals and Alloys. Metallurg. Reviews 6 (1961)
Nesmeyanow, A., N.: Vapor Pressure of the Chemical Elements: Elsevier, Amsterdam-London-New York, 1963
Wyckoff, R., W., G.: Crystal Structures. Vol 1,New York, 1963




