Difference between revisions of "Precipitation Hardening Copper Alloys"
(→5.1.6.2.1 Copper-Chromium Alloys) |
Doduco Admin (talk | contribs) (→Other Precipitation Hardening Copper Alloys) |
||
| (39 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | Besides the naturally hard copper materials, precipitation hardening copper alloys play also an important role as carrier materials for electrical contacts. By means of a suitable heat treatment, finely dispersed precipitations of a second phase can be achieved, that increases the mechanical strength of these copper alloys significantly. | |
| − | + | ====<!--5.1.6.1-->Copper-Beryllium Alloys (Beryllium Bronze)==== | |
| − | |||
| + | The cause for precipitation hardening of CuBe materials, is the rapidly diminishing solubility of beryllium in copper as temperature decreases. As the | ||
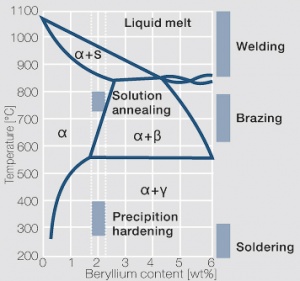
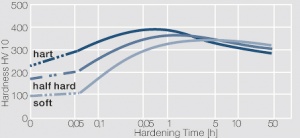
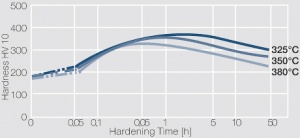
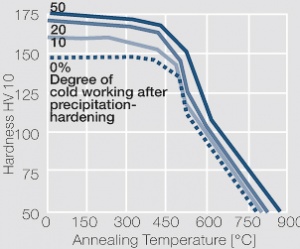
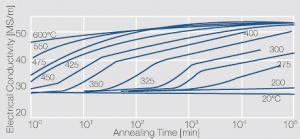
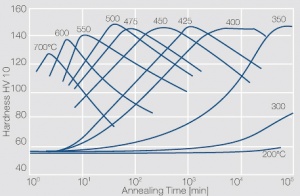
| + | phase diagram for CuBe shows, 2.4 wt% of Be are soluble in Cu at 780°C (<xr id="fig:Phase_diagram_of_copperberyllium_with_temperature_ranges_for_brazing_and_annealing_treatments"/><!--(Fig. 5.28)-->). In this temperature range, annealed CuBe alloys are homogeneous(solution annealing). The homogeneous state can be frozen through rapid cooling to room temperature (quenching). Through a subsequent annealing at 325°C, the desired precipitation hardening is achieved, which results in a significant increase in mechanical strength and electrical conductivity of CuBe (<xr id="tab:Physical_Properties_of_Selected_Copper_Beryllium_Alloys"/><!--(Tab. 5.17)-->). The final strength and hardness values depend on the annealing temperature and time, as well as on the initial degree of cold working (<xr id="tab:Mechanical Properties of Selected Copper-Beryllium Alloys"/><!--(Table 5.18)--> and <xr id="fig:Precipitation_hardening_of_CuBe2_at_325°C_after_different_cold_working"/>, <xr id="fig:Precipitation_hardening_of_CuBe2_(soft)_at_325°C"/>, <xr id="fig:Precipitation_hardening_of_CuBe2_(half hard)_at_different_annealing_temperatures"/>). | ||
| − | |||
| − | |||
| − | + | As precipitation hardening alloys CuBe materials, mainly CuBe2 and CuBe1.7 have gained broad usage as current carrying contact springs because of their outstanding mechanical properties. Besides these, CuCo2Be and CuNi2Be, which have medium mechanical strength and a relatively high electrical | |
| + | conductivity, are also used as contact carrier materials. After stamping and forming into desired contact configurations, these CuBe materials are then precipitation hardened. CuBe alloys are available as semi-finished materials in a variety of cold work conditions. They can also be supplied and used in the already precipitation hardened condition, without significant strength losses. In this case, the hardening was already performed at the alloy producer. | ||
| − | + | Since Beryllium is rated as a carcinogen by the European regulation EU-67/548, it has been tried to reach the application properties of the well established CuBe1.7 and CuBe2 alloys with a lower Be content. Development efforts for alternative precipitation hardening materials without toxic and declarable additives are underway, e.g. CuNiCoSi as a substitute for CuBe. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<div class="multiple-images"> | <div class="multiple-images"> | ||
| − | <figure id="fig: | + | <figure id="fig:Phase_diagram_of_copperberyllium_with_temperature_ranges_for_brazing_and_annealing_treatments"> |
[[File:Phase diagram of copper beryllium with temperature ranges.jpg|left|thumb|<caption>Phase diagram of copper- beryllium with temperature ranges for brazing and annealing treatments</caption>]] | [[File:Phase diagram of copper beryllium with temperature ranges.jpg|left|thumb|<caption>Phase diagram of copper- beryllium with temperature ranges for brazing and annealing treatments</caption>]] | ||
</figure> | </figure> | ||
| − | <figure id="fig: | + | <figure id="fig:Precipitation_hardening_of_CuBe2_at_325°C_after_different_cold_working"> |
[[File:Precipitation hardening of CuBe2 at 325C.jpg|left|thumb|<caption>Precipitation hardening of CuBe2 at 325°C after different cold working</caption>]] | [[File:Precipitation hardening of CuBe2 at 325C.jpg|left|thumb|<caption>Precipitation hardening of CuBe2 at 325°C after different cold working</caption>]] | ||
</figure> | </figure> | ||
| − | <figure id="fig: | + | <figure id="fig:Precipitation_hardening_of_CuBe2_(soft)_at_325°C"> |
[[File:Precipitation hardening of CuBe2 (soft) at 325C.jpg|left|thumb|<caption>Precipitation hardening of CuBe2 (soft) at 325°C</caption>]] | [[File:Precipitation hardening of CuBe2 (soft) at 325C.jpg|left|thumb|<caption>Precipitation hardening of CuBe2 (soft) at 325°C</caption>]] | ||
</figure> | </figure> | ||
| − | <figure id="fig: | + | <figure id="fig:Precipitation_hardening_of_CuBe2_(half hard)_at_different_annealing_temperatures"> |
[[File:Precipitation hardening of CuBe2 half hard.jpg|left|thumb|<caption>Precipitation hardening of CuBe2 (half hard) at different annealing temperatures</caption>]] | [[File:Precipitation hardening of CuBe2 half hard.jpg|left|thumb|<caption>Precipitation hardening of CuBe2 (half hard) at different annealing temperatures</caption>]] | ||
</figure> | </figure> | ||
| Line 42: | Line 33: | ||
| − | + | <figtable id="tab:Physical_Properties_of_Selected_Copper_Beryllium_Alloys"> | |
| − | <figtable id="tab: | + | <caption>'''<!--Table 5.17:-->Physical Properties of Selected Copper-Beryllium Alloys'''</caption> |
| − | '''Table 5.17: Physical Properties of Selected Copper-Beryllium Alloys''' | ||
{| class="twocolortable" style="text-align: left; font-size: 12px" | {| class="twocolortable" style="text-align: left; font-size: 12px" | ||
| Line 51: | Line 41: | ||
!Composition<br />[wt%] | !Composition<br />[wt%] | ||
!Density<br />[g/cm<sup>3</sup>] | !Density<br />[g/cm<sup>3</sup>] | ||
| − | !colspan="2" style="text-align:center"|Electrical<br />Conductivity | + | !colspan="2" style="text-align:center"|Electrical<br />Conductivity |
!Electrical<br />Resistivity<br />[μΩ·cm] | !Electrical<br />Resistivity<br />[μΩ·cm] | ||
!Thermal<br />Conductivity<br />[W/(m·K)] | !Thermal<br />Conductivity<br />[W/(m·K)] | ||
| Line 58: | Line 48: | ||
!Softening Temperature<br />(approx. 10% loss in<br />strength)<br />[°C] | !Softening Temperature<br />(approx. 10% loss in<br />strength)<br />[°C] | ||
!Melting<br />Temp Range<br />[°C] | !Melting<br />Temp Range<br />[°C] | ||
| + | |- | ||
| + | ! | ||
| + | ! | ||
| + | ! | ||
| + | ![MS/m] | ||
| + | ![% IACS] | ||
| + | ! | ||
| + | ! | ||
| + | ! | ||
| + | ! | ||
| + | ! | ||
| + | ! | ||
|- | |- | ||
|CuBe1.7<br />CW100C<br />C17000 | |CuBe1.7<br />CW100C<br />C17000 | ||
|Be 1.6 - 1.8<br />Co 0.3<br />Ni 0.3<br />Cu Rest | |Be 1.6 - 1.8<br />Co 0.3<br />Ni 0.3<br />Cu Rest | ||
|8.4 | |8.4 | ||
| − | |8 - 9<sup>a</sup><br />12 - 13<sup>b</sup><br />11<sup>c</sup> | + | |8 - 9[[#text-reference1|<sup>a</sup>]]<br />12 - 13[[#text-reference2|<sup>b</sup>]]<br />11[[#text-reference3|<sup>c</sup>]] |
|14 - 16<br />21 - 22<br />19 | |14 - 16<br />21 - 22<br />19 | ||
| − | |11 - 12.5<sup>a</sup><br />7.7 - 8.3<sup>b</sup> | + | |11 - 12.5[[#text-reference1|<sup>a</sup>]]<br />7.7 - 8.3[[#text-reference2|<sup>b</sup>]]9.1[[#text-reference3|<sup>c</sup>]] |
|110 | |110 | ||
|17 | |17 | ||
| − | |125<sup>a</sup><br /> | + | |125[[#text-reference1|<sup>a</sup>]]<br />[[#text-reference2|<sup>b</sup>]] |
|ca. 380 | |ca. 380 | ||
|890 - 1000 | |890 - 1000 | ||
| Line 74: | Line 76: | ||
|Be 1.8 - 2.1<br />Co 0.3<br />Ni 0.3<br />Cu Rest | |Be 1.8 - 2.1<br />Co 0.3<br />Ni 0.3<br />Cu Rest | ||
|8.3 | |8.3 | ||
| − | |8 - 9<sup>a</sup><br />12 - 13<sup>b</sup><br />11<sup>c</sup> | + | |8 - 9[[#text-reference1|<sup>a</sup>]]<br />12 - 13[[#text-reference2|<sup>b</sup>]]<br />11[[#text-reference3|<sup>c</sup>]] |
|14 - 16<br />21 - 22<br />19 | |14 - 16<br />21 - 22<br />19 | ||
| − | |11 - 12.5<sup>a</sup><br />7.7 - 8.3<sup>b</sup><br />9.1<sup>c</sup> | + | |11 - 12.5[[#text-reference1|<sup>a</sup>]]<br />7.7 - 8.3[[#text-reference2|<sup>b</sup>]]<br />9.1[[#text-reference3|<sup>c</sup>]] |
|110 | |110 | ||
|17 | |17 | ||
| − | |125<sup>a</sup><br />135<sup>b</sup> | + | |125[[#text-reference1|<sup>a</sup>]]<br />135[[#text-reference2|<sup>b</sup>]] |
|ca. 380 | |ca. 380 | ||
|870 - 980 | |870 - 980 | ||
| Line 86: | Line 88: | ||
|Co 2.0 - 2.8<br />Be 0.4 - 0.7<br />Ni 0.3<br />Cu Rest | |Co 2.0 - 2.8<br />Be 0.4 - 0.7<br />Ni 0.3<br />Cu Rest | ||
|8.8 | |8.8 | ||
| − | |11 - 14<sup>a</sup><br />25 - 27<sup>b</sup><br />27 - 34<sup>c</sup> | + | |11 - 14[[#text-reference1|<sup>a</sup>]]<br />25 - 27[[#text-reference2|<sup>b</sup>]]<br />27 - 34[[#text-reference3|<sup>c</sup>]] |
|19 - 24<br />43 - 47<br />47 - 59 | |19 - 24<br />43 - 47<br />47 - 59 | ||
| − | |7.1 - 9.1<sup>a</sup><br />3.7 - 4.0<sup>b</sup><br />2.9<sup>c</sup> | + | |7.1 - 9.1[[#text-reference1|<sup>a</sup>]]<br />3.7 - 4.0[[#text-reference2|<sup>b</sup>]]<br />2.9[[#text-reference3|<sup>c</sup>]] |
|210 | |210 | ||
|18 | |18 | ||
| − | |131<sup>a</sup><br />138<sup>b</sup> | + | |131[[#text-reference1|<sup>a</sup>]]<br />138[[#text-reference2|<sup>b</sup>]] |
|ca. 450 | |ca. 450 | ||
|1030 - 1070 | |1030 - 1070 | ||
| Line 98: | Line 100: | ||
|Ni 1.4 - 2.2<br />Be 0.2 - 0.6<br />Co 0.3<br />Cu Rest | |Ni 1.4 - 2.2<br />Be 0.2 - 0.6<br />Co 0.3<br />Cu Rest | ||
|8.8 | |8.8 | ||
| − | |11 - 14<sup>a</sup><br />25 - 27<sup>b</sup><br />27 - 34<sup>c</sup> | + | |11 - 14[[#text-reference1|<sup>a</sup>]]<br />25 - 27[[#text-reference2|<sup>b</sup>]]<br />27 - 34[[#text-reference3|<sup>c</sup>]] |
|19 - 24<br />43 - 47<br />47 - 59 | |19 - 24<br />43 - 47<br />47 - 59 | ||
| − | |7.1 - 9.1<sup>a</sup><br />3.7 - 4.0<sup>b</sup><br />2.9<sup>c</sup> | + | |7.1 - 9.1[[#text-reference1|<sup>a</sup>]]<br />3.7 - 4.0[[#text-reference2|<sup>b</sup>]]<br />2.9[[#text-reference3|<sup>c</sup>]] |
|230 | |230 | ||
|18 | |18 | ||
| − | |131<sup>a</sup><br />138<sup>b</sup> | + | |131[[#text-reference1|<sup>a</sup>]]<br />138[[#text-reference2|<sup>b</sup>]] |
|ca. 480 | |ca. 480 | ||
|1060 - 1100 | |1060 - 1100 | ||
|} | |} | ||
| + | <div id="text-reference1"><sub>a</sub> solution annealed, and cold rolled</div> | ||
| + | <div id="text-reference2"><sub>b</sub> solution annealed, cold rolled, and precipitation hardened</div> | ||
| + | <div id="text-reference3"><sub>c</sub> solution annealed, cold rolled, and precipitation hardened at mill (mill hardened)</div> | ||
</figtable> | </figtable> | ||
| + | <br/> | ||
| + | <br/> | ||
| − | + | <figtable id="tab:Mechanical Properties of Selected Copper-Beryllium Alloys"> | |
| − | + | <caption>'''<!--Table 5.18:-->Mechanical Properties of Selected Copper-Beryllium Alloys'''</caption> | |
| − | |||
| − | |||
| − | |||
| − | <figtable id="tab: | ||
| − | '''Table 5.18: Mechanical Properties of Selected Copper-Beryllium Alloys''' | ||
{| class="twocolortable" style="text-align: left; font-size: 12px" | {| class="twocolortable" style="text-align: left; font-size: 12px" | ||
| Line 125: | Line 127: | ||
!Elongation<br />A<sub>50</sub><br />[%] | !Elongation<br />A<sub>50</sub><br />[%] | ||
!Vickers<br />Hardness<br />HV | !Vickers<br />Hardness<br />HV | ||
| − | !Bend Radius<sup>1 | + | !Bend Radius[[#text-reference4|<sup>1</sup>]]<br />perpendicular to<br />rolling direction |
| − | !Bend Radius<sup>1 | + | !Bend Radius[[#text-reference4|<sup>1</sup>]]<br />parallel to<br />rolling direction |
!Spring Bending<br />Limit σ<sub>FB</sub><br />[MPa] | !Spring Bending<br />Limit σ<sub>FB</sub><br />[MPa] | ||
!Spring Fatigue<br />Limit σ<sub>BW</sub><br />[MPa] | !Spring Fatigue<br />Limit σ<sub>BW</sub><br />[MPa] | ||
|- | |- | ||
|CuBe1,7 | |CuBe1,7 | ||
| − | |R 390<sup>a</sup><br />R 680<sup>a</sup><br />R 1030<sup>b</sup><br />R 1240<sup>b</sup><br />R 680<sup>c</sup><br />R 1100<sup>c</sup> | + | |R 390[[#text-reference5|<sup>a</sup>]]<br />R 680[[#text-reference5|<sup>a</sup>]]<br />R 1030[[#text-reference6|<sup>b</sup>]]<br />R 1240[[#text-reference6|<sup>b</sup>]]<br />R 680[[#text-reference7|<sup>c</sup>]]<br />R 1100[[#text-reference7|<sup>c</sup>]] |
|380 -520<br />680 - 820<br />1030 - 1240<br />1240 - 1380<br />680 - 750<br />1100 - 1200 | |380 -520<br />680 - 820<br />1030 - 1240<br />1240 - 1380<br />680 - 750<br />1100 - 1200 | ||
|≥ 180<br />≥ 600<br />≥ 900<br />≥ 1070<br />≥ 480<br />≥ 930 | |≥ 180<br />≥ 600<br />≥ 900<br />≥ 1070<br />≥ 480<br />≥ 930 | ||
| Line 142: | Line 144: | ||
|- | |- | ||
|CuBe2 | |CuBe2 | ||
| − | |R 410<sup>a</sup><br />R 690<sup>a</sup><br />R 1140<sup>b</sup><br />R 1310<sup>b</sup><br />R 690<sup>c</sup><br />R 1200<sup>c</sup> | + | |R 410[[#text-reference5|<sup>a</sup>]]<br />R 690[[#text-reference5|<sup>a</sup>]]<br />R 1140[[#text-reference6|<sup>b</sup>]]<br />R 1310[[#text-reference6|<sup>b</sup>]]<br />R 690[[#text-reference7|<sup>c</sup>]]<br />R 1200[[#text-reference7|<sup>c</sup>]] |
|410 -540<br />690 - 820<br />1140 - 1310<br />1310 - 1480<br />690 - 760<br />1200 - 1320 | |410 -540<br />690 - 820<br />1140 - 1310<br />1310 - 1480<br />690 - 760<br />1200 - 1320 | ||
|≥ 190<br />≥ 650<br />≥ 1000<br />≥ 1150<br />≥ 480<br />≥ 1030 | |≥ 190<br />≥ 650<br />≥ 1000<br />≥ 1150<br />≥ 480<br />≥ 1030 | ||
| Line 153: | Line 155: | ||
|- | |- | ||
|CuCo2Be<br />CuNi2Be | |CuCo2Be<br />CuNi2Be | ||
| − | |R 250<sup>a</sup><br />R 550<sup>a</sup><br />R 650<sup>b</sup><br />R 850<sup>b</sup><br />R 520<sup>c</sup> | + | |R 250[[#text-reference5|<sup>a</sup>]]<br />R 550[[#text-reference5|<sup>a</sup>]]<br />R 650[[#text-reference6|<sup>b</sup>]]<br />R 850[[#text-reference6|<sup>b</sup>]]<br />R 520[[#text-reference7|<sup>c</sup>]] |
|250 - 380<br />550 - 700<br />650 - 820<br />850 - 1000<br />520 - 620 | |250 - 380<br />550 - 700<br />650 - 820<br />850 - 1000<br />520 - 620 | ||
|≥ 140<br />≥ 450<br />≥ 520<br />≥ 750<br />≥ 340 | |≥ 140<br />≥ 450<br />≥ 520<br />≥ 750<br />≥ 340 | ||
| Line 163: | Line 165: | ||
| <br /> <br />220<br />250<br />210 | | <br /> <br />220<br />250<br />210 | ||
|} | |} | ||
| − | < | + | <div id="text-reference4"><sub>1</sub> t: Strip thickness max. 0.5 mm</div> |
| − | < | + | <div id="text-reference5"><sub>a</sub> solution annealed, and cold rolled</div> |
| − | < | + | <div id="text-reference6"><sub>b</sub> solution annealed, cold rolled, and precipitation hardened</div> |
| − | < | + | <div id="text-reference7"><sub>c</sub> solution annealed, cold rolled, and precipitation hardened at mill (mill hardened)</div> |
| − | < | + | </figtable> |
| − | + | <br/> | |
| − | + | <br/> | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | < | ||
| − | |||
| − | < | ||
| − | < | + | ====<!--5.1.6.2-->Other Precipitation Hardening Copper Alloys==== |
| − | < | + | =====<!--5.1.6.2.1-->Copper-Chromium Alloys===== |
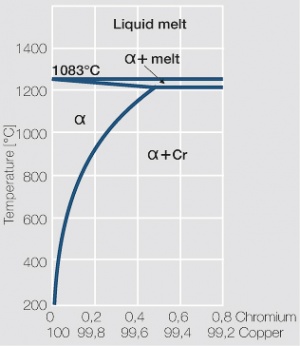
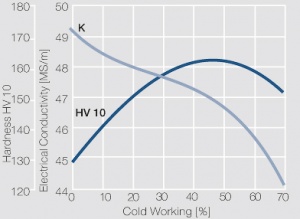
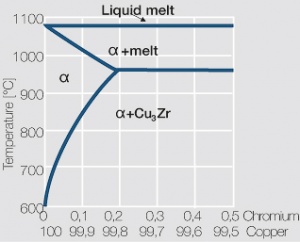
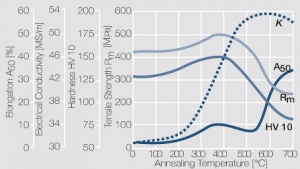
| − | <xr id="fig: | + | As the phase diagram shows, copper-chromium has a similar hardening profile compared to CuBe (<xr id="fig:Copper corner of the copper-chromium phase diagram for up to 0.8 wt% chromium"/><!--(Fig. 5.32)-->). In the hardened stage CuCr has limitations to work hardening. Compared to copper it has a better temperature stability with good electrical conductivity. Hardness and electrical conductivity as a function of cold working and precipitation hardening conditions are illustrated in [[#figures8|(Figs. 6 – 9]]<!--Figs. 5.33-5.35--> and <xr id="tab:Physical Properties of Other Precipitation Hardening Copper Alloys"/><!--(Tables 5.19)--> and <xr id="tab:Mechanical Properties of Other Precipitation Hardening Copper Alloys"/><!--(Tab. 5.20)-->). |
| − | + | Copper-chromium materials are especially suitable for use as electrodes for resistance welding. During brazing the loss in hardness is limited, if low melting brazing alloys and reasonably short heating times are used. | |
| − | |||
<div class="multiple-images"> | <div class="multiple-images"> | ||
| Line 216: | Line 206: | ||
| − | + | <figtable id="tab:Physical Properties of Other Precipitation Hardening Copper Alloys"> | |
| − | <figtable id="tab: | + | <caption>'''<!--Table 5.19:-->Physical Properties of Other Precipitation Hardening Copper Alloys'''</caption> |
| − | '''Table 5.19: Physical Properties of Other Precipitation Hardening Copper Alloys''' | ||
{| class="twocolortable" style="text-align: left; font-size: 12px" | {| class="twocolortable" style="text-align: left; font-size: 12px" | ||
| Line 225: | Line 214: | ||
!Composition<br />[wt%] | !Composition<br />[wt%] | ||
!Density<br />[g/cm<sup>3</sup>] | !Density<br />[g/cm<sup>3</sup>] | ||
| − | !colspan="2" style="text-align:center"|Electrical<br />Conductivity | + | !colspan="2" style="text-align:center"|Electrical<br />Conductivity |
!Electrical<br />Resistivity<br />[μΩ·cm] | !Electrical<br />Resistivity<br />[μΩ·cm] | ||
!Thermal<br />Conductivity<br />[W/(m·K)] | !Thermal<br />Conductivity<br />[W/(m·K)] | ||
| Line 233: | Line 222: | ||
!Melting<br />Temp Range<br />[°C] | !Melting<br />Temp Range<br />[°C] | ||
|- | |- | ||
| − | + | ! | |
| − | + | ! | |
| − | + | ! | |
| − | + | ![MS/m] | |
| − | + | ![% IACS] | |
| − | + | ! | |
| − | + | ! | |
| − | + | ! | |
| − | + | ! | |
| − | + | ! | |
| − | + | ! | |
|- | |- | ||
| − | | | + | |CuCr |
| − | | | + | |Cr 0.3 - 1.2<br />Cu Rest |
| − | |8. | + | |8.89 |
| − | | | + | |26[[#text-reference8|<sup>a</sup>]]<br />48[[#text-reference9|<sup>b</sup>]] |
| − | + | |45[[#text-reference8|<sup>a</sup>]]<br />83[[#text-reference9|<sup>b</sup>]] | |
| − | | | + | |3.8[[#text-reference8|<sup>a</sup>]]<br />2.1[[#text-reference9|<sup>b</sup>]] |
| − | + | |170[[#text-reference8|<sup>a</sup>]]<br />315[[#text-reference9|<sup>b</sup>]] | |
|17 | |17 | ||
| − | | | + | |112 |
| − | |ca. | + | |ca. 450 |
| − | | | + | |980 - 1080 |
|- | |- | ||
| − | | | + | |CuZr |
| − | | | + | |Zr 0.1 - 0.3<br />Cu Rest |
| − | |8. | + | |8.9 |
| − | | | + | |35[[#text-reference8|<sup>a</sup>]]<br />52[[#text-reference9|<sup>b</sup>]] |
| − | + | |60[[#text-reference8|<sup>a</sup>]]<br />90[[#text-reference9|<sup>b</sup>]] | |
| − | | | + | |2.9[[#text-reference8|<sup>a</sup>]]<br />1.9[[#text-reference9|<sup>b</sup>]] |
| − | | | + | |340[[#text-reference8|<sup>a</sup>]] |
| − | + | |16 | |
| − | | | + | |135 |
| − | |ca. | + | |ca. 500 |
| − | | | + | |1020 - 1080 |
|- | |- | ||
| − | | | + | |CuCr1Zr<br />CW106C<br />C18150 |
| − | | | + | |Cr 0.5 - 1.2<br />Zr 0.03 - 0.3<br />Cu Rest |
| − | |8. | + | |8.92 |
| − | | | + | |20[[#text-reference8|<sup>a</sup>]]<br />43[[#text-reference9|<sup>b</sup>]] |
| − | + | |34[[#text-reference8|<sup>a</sup>]]<br />74[[#text-reference9|<sup>b</sup>]] | |
| − | | | + | |5.0[[#text-reference8|<sup>a</sup>]]<br />2.3[[#text-reference9|<sup>b</sup>]] |
| − | | | + | |170[[#text-reference8|<sup>a</sup>]]<br />310 - 330[[#text-reference9|<sup>b</sup>]] |
| − | | | + | |16 |
| − | | | + | |110[[#text-reference8|<sup>a</sup>]]<br />130[[#text-reference9|<sup>b</sup>]] |
| − | |ca. | + | |ca. 500 |
| − | | | + | |1070 - 1080 |
|} | |} | ||
| + | <div id="text-reference8"><sub>a</sub> solution annealed, and cold rolled</div> | ||
| + | <div id="text-reference9"><sub>b</sub> solution annealed, cold rolled, and precipitation hardened</div> | ||
</figtable> | </figtable> | ||
| + | <br /> | ||
| + | <br /> | ||
| + | |||
| + | <figtable id="tab:Mechanical Properties of Other Precipitation Hardening Copper Alloys"> | ||
| + | <caption>'''<!--Table 5.20:-->Mechanical Properties of Other Precipitation Hardening Copper Alloys'''</caption> | ||
| − | < | + | <table class="twocolortable"> |
| − | < | + | <tr><th><p class="s16">Material</p></th><th><p class="s16">Hardness</p><p class="s16">Condi- tion</p></th><th><p class="s16">Tensile</p><p class="s16">Strength R<span class="s18">m</span></p><p class="s16">[MPa]</p></th><th><p class="s16">0,2% Yield</p><p class="s16">Strength R<span class="s18">p02</span></p><p class="s16">[MPa]</p></th><th><p class="s16">Elongation</p><p class="s16">A50</p><p class="s16">[%]</p></th><th><p class="s16">Vickers</p><p class="s16">Hardness</p><p class="s16">HV</p></th><th><p class="s16">Spring Bending</p><p class="s16">Limit <span class="s19">F</span><span class="s18">FB </span>[MPa]</p></th></tr><tr><td><p class="s16">CuCr</p></td><td><p class="s16">R 230<span class="s18">a</span></p><p class="s16">R 400<span class="s18">a </span>R 450<span class="s18">b </span>R 550<span class="s18">b</span></p></td><td><p class="s33">><span class="s16"> 230</span></p><p class="s33">><span class="s16"> 400</span></p><p class="s33">><span class="s16"> 450</span></p><p class="s33">><span class="s16"> 550</span></p></td><td><p class="s33">><span class="s16"> 80</span></p><p class="s33">><span class="s16"> 295</span></p><p class="s33">><span class="s16"> 325</span></p><p class="s33">><span class="s16"> 440</span></p></td><td><p class="s16">30</p><p class="s16">10</p><p class="s16">10</p><p class="s16">8</p></td><td><p class="s33">><span class="s16"> 55</span></p><p class="s33">><span class="s16"> 120</span></p><p class="s33">><span class="s16"> 130</span></p><p class="s33">><span class="s16"> 150</span></p></td><td><p class="s16">350</p></td></tr><tr><td><p class="s16">CuZr</p></td><td><p class="s16">R 260<span class="s18">a</span></p><p class="s16">R 370<span class="s18">a </span>R 400<span class="s18">b </span>R 420<span class="s18">b</span></p></td><td><p class="s33">><span class="s16"> 260</span></p><p class="s33">><span class="s16"> 370</span></p><p class="s33">><span class="s16"> 400</span></p><p class="s33">><span class="s16"> 420</span></p></td><td><p class="s33">><span class="s16"> 100</span></p><p class="s33">><span class="s16"> 270</span></p><p class="s33">><span class="s16"> 280</span></p><p class="s33">><span class="s16"> 400</span></p></td><td><p class="s16">35</p><p class="s16">12</p><p class="s16">12</p><p class="s16">10</p></td><td><p class="s33">><span class="s16"> 55</span></p><p class="s33">><span class="s16"> 100</span></p><p class="s33">><span class="s16"> 105</span></p><p class="s33">><span class="s16"> 115</span></p></td><td><p class="s16">280</p></td></tr><tr><td><p class="s16">CuCr1Zr</p></td><td><p class="s16">R 200<span class="s18">a</span></p><p class="s16">R 400<span class="s18">b</span></p><p class="s16">R 450<span class="s18">b</span></p></td><td><p class="s33">><span class="s16"> 200</span></p><p class="s33">><span class="s16"> 400</span></p><p class="s33">><span class="s16"> 450</span></p></td><td><p class="s33">><span class="s16"> 60</span></p><p class="s33">><span class="s16"> 210</span></p><p class="s33">><span class="s16"> 360</span></p></td><td><p class="s16">30</p><p class="s16">12</p><p class="s16">10</p></td><td><p class="s33">><span class="s16"> 70</span></p><p class="s33">><span class="s16"> 140</span></p><p class="s33">><span class="s16"> 155</span></p></td><td><p class="s16">420</p></td></tr></table> |
| + | </figtable> | ||
| − | < | + | =====<!--5.1.6.2.2-->Copper-Zirconium Alloys===== |
| − | |||
| − | < | + | The solubility of Zirconium in copper is 0.15 wt% Zr at the eutectic temperature of 980°C (<xr id="fig:Copper corner of the copper zirconium for up to 0.5-wt zirconium"/><!--(Fig. 5.36)-->). Copper-zirconium materials have a similar properties spectrum, compared to the one for copper-chromium materials. At room temperature the mechanical properties of copper-zirconium are less suitable than those of copper chromium, its temperature stability is however at least the same. |
| − | |||
| − | =====5.1.6.2. | + | =====<!--5.1.6.2.3-->Copper-Chromium-Zirconium Alloys===== |
| − | The | + | The earlier used CuCr and CuZr materials have been partially replaced over the years, by the capitation hardening three materials alloy CuCr1Zr. This material exhibits high mechanical strength at elevated temperatures and good oxidation resistance as well as high softening temperatures. In its hardened condition CuCr1Zr has also a high electrical conductivity (<xr id="fig:Softening of CuCr1Zr after 1hr annealing"/><!--(Bild 5.37)-->). Their usage extends from mechanically and thermally highly stressed parts, such as contact tulips in high voltage switchgear or electrodes for resistance welding. |
| + | <div class="multiple-images"> | ||
<figure id="fig:Copper corner of the copper zirconium for up to 0.5-wt zirconium"> | <figure id="fig:Copper corner of the copper zirconium for up to 0.5-wt zirconium"> | ||
| − | [[File:Copper corner of the copper zirconium for up to 0.5-wt zirconium.jpg|right|thumb|Copper corner of the copper- zirconium for up to 0.5 wt% zirconium]] | + | [[File:Copper corner of the copper zirconium for up to 0.5-wt zirconium.jpg|right|thumb|Figure 10: Copper corner of the copper- zirconium for up to 0.5 wt% zirconium]] |
</figure> | </figure> | ||
| − | |||
| − | |||
| − | |||
| − | |||
<figure id="fig:Softening of CuCr1Zr after 1hr annealing"> | <figure id="fig:Softening of CuCr1Zr after 1hr annealing"> | ||
| − | [[File:Softening of CuCr1Zr after 1hr annealing.jpg|right|thumb|Softening of CuCr1Zr after 1 hr annealing and after 90% cold working]] | + | [[File:Softening of CuCr1Zr after 1hr annealing.jpg|right|thumb|Figure 11: Softening of CuCr1Zr after 1 hr annealing and after 90% cold working]] |
</figure> | </figure> | ||
| + | </div> | ||
| + | <div class="clear"></div> | ||
==References== | ==References== | ||
[[Contact Carrier Materials#References|References]] | [[Contact Carrier Materials#References|References]] | ||
| + | |||
| + | [[de:Aushärtbare_Kupfer-Legierungen]] | ||
Latest revision as of 07:58, 12 January 2023
Besides the naturally hard copper materials, precipitation hardening copper alloys play also an important role as carrier materials for electrical contacts. By means of a suitable heat treatment, finely dispersed precipitations of a second phase can be achieved, that increases the mechanical strength of these copper alloys significantly.
Contents
Copper-Beryllium Alloys (Beryllium Bronze)
The cause for precipitation hardening of CuBe materials, is the rapidly diminishing solubility of beryllium in copper as temperature decreases. As the phase diagram for CuBe shows, 2.4 wt% of Be are soluble in Cu at 780°C (Figure 1). In this temperature range, annealed CuBe alloys are homogeneous(solution annealing). The homogeneous state can be frozen through rapid cooling to room temperature (quenching). Through a subsequent annealing at 325°C, the desired precipitation hardening is achieved, which results in a significant increase in mechanical strength and electrical conductivity of CuBe (Table 1). The final strength and hardness values depend on the annealing temperature and time, as well as on the initial degree of cold working (Table 2 and Figure 2, Figure 3, Figure 4).
As precipitation hardening alloys CuBe materials, mainly CuBe2 and CuBe1.7 have gained broad usage as current carrying contact springs because of their outstanding mechanical properties. Besides these, CuCo2Be and CuNi2Be, which have medium mechanical strength and a relatively high electrical
conductivity, are also used as contact carrier materials. After stamping and forming into desired contact configurations, these CuBe materials are then precipitation hardened. CuBe alloys are available as semi-finished materials in a variety of cold work conditions. They can also be supplied and used in the already precipitation hardened condition, without significant strength losses. In this case, the hardening was already performed at the alloy producer.
Since Beryllium is rated as a carcinogen by the European regulation EU-67/548, it has been tried to reach the application properties of the well established CuBe1.7 and CuBe2 alloys with a lower Be content. Development efforts for alternative precipitation hardening materials without toxic and declarable additives are underway, e.g. CuNiCoSi as a substitute for CuBe.
| Material Designation EN UNS |
Composition [wt%] |
Density [g/cm3] |
Electrical Conductivity |
Electrical Resistivity [μΩ·cm] |
Thermal Conductivity [W/(m·K)] |
Coeff. of Linear Thermal Expansion [10-6/K] |
Modulus of Elasticity [GPa] |
Softening Temperature (approx. 10% loss in strength) [°C] |
Melting Temp Range [°C] | |
|---|---|---|---|---|---|---|---|---|---|---|
| [MS/m] | [% IACS] | |||||||||
| CuBe1.7 CW100C C17000 |
Be 1.6 - 1.8 Co 0.3 Ni 0.3 Cu Rest |
8.4 | 8 - 9a 12 - 13b 11c |
14 - 16 21 - 22 19 |
11 - 12.5a 7.7 - 8.3b9.1c |
110 | 17 | 125a b |
ca. 380 | 890 - 1000 |
| CuBe2 CW101C C17200 |
Be 1.8 - 2.1 Co 0.3 Ni 0.3 Cu Rest |
8.3 | 8 - 9a 12 - 13b 11c |
14 - 16 21 - 22 19 |
11 - 12.5a 7.7 - 8.3b 9.1c |
110 | 17 | 125a 135b |
ca. 380 | 870 - 980 |
| CuCo2Be CW104C C17500 |
Co 2.0 - 2.8 Be 0.4 - 0.7 Ni 0.3 Cu Rest |
8.8 | 11 - 14a 25 - 27b 27 - 34c |
19 - 24 43 - 47 47 - 59 |
7.1 - 9.1a 3.7 - 4.0b 2.9c |
210 | 18 | 131a 138b |
ca. 450 | 1030 - 1070 |
| CuNi2Be CW110C C17510 |
Ni 1.4 - 2.2 Be 0.2 - 0.6 Co 0.3 Cu Rest |
8.8 | 11 - 14a 25 - 27b 27 - 34c |
19 - 24 43 - 47 47 - 59 |
7.1 - 9.1a 3.7 - 4.0b 2.9c |
230 | 18 | 131a 138b |
ca. 480 | 1060 - 1100 |
| Material | Hardness Condition |
Tensile Strength Rm [MPa] |
0,2% Yield Strength Rp02 [MPa] |
Elongation A50 [%] |
Vickers Hardness HV |
Bend Radius1 perpendicular to rolling direction |
Bend Radius1 parallel to rolling direction |
Spring Bending Limit σFB [MPa] |
Spring Fatigue Limit σBW [MPa] |
|---|---|---|---|---|---|---|---|---|---|
| CuBe1,7 | R 390a R 680a R 1030b R 1240b R 680c R 1100c |
380 -520 680 - 820 1030 - 1240 1240 - 1380 680 - 750 1100 - 1200 |
≥ 180 ≥ 600 ≥ 900 ≥ 1070 ≥ 480 ≥ 930 |
35 2 3 1 18 3 |
80 - 135 210 - 250 330 - 380 360 - 420 220 - 350 330 - 370 |
0 x t 1 x t 1 x t 1 x t 6 x t |
0 x t 3 x t 1.5 x t 1 x t 10 x t |
700 1000 390 790 |
260 280 260 |
| CuBe2 | R 410a R 690a R 1140b R 1310b R 690c R 1200c |
410 -540 690 - 820 1140 - 1310 1310 - 1480 690 - 760 1200 - 1320 |
≥ 190 ≥ 650 ≥ 1000 ≥ 1150 ≥ 480 ≥ 1030 |
35 2 3 1 18 3 |
90 - 140 215 - 260 350 - 400 380 - 450 220 - 250 360 - 410 |
0 x t 1 x t 1 x t 5 x t |
0 x t 3 x t 1.5 x t 10 x t |
800 1040 400 900 |
270 300 280 |
| CuCo2Be CuNi2Be |
R 250a R 550a R 650b R 850b R 520c |
250 - 380 550 - 700 650 - 820 850 - 1000 520 - 620 |
≥ 140 ≥ 450 ≥ 520 ≥ 750 ≥ 340 |
20 2 10 1 5 |
60 - 90 160 - 200 195 - 230 240 - 290 150 - 180 |
0 x t 3 x t 1 x t 3 x t 1 x t |
0 x t 1 x t 3.5 x t 1 x t |
360 650 300 |
220 250 210 |
Other Precipitation Hardening Copper Alloys
Copper-Chromium Alloys
As the phase diagram shows, copper-chromium has a similar hardening profile compared to CuBe (Figure 5). In the hardened stage CuCr has limitations to work hardening. Compared to copper it has a better temperature stability with good electrical conductivity. Hardness and electrical conductivity as a function of cold working and precipitation hardening conditions are illustrated in (Figs. 6 – 9 and Table 3 and Table 4).
Copper-chromium materials are especially suitable for use as electrodes for resistance welding. During brazing the loss in hardness is limited, if low melting brazing alloys and reasonably short heating times are used.
| Material Designation EN UNS |
Composition [wt%] |
Density [g/cm3] |
Electrical Conductivity |
Electrical Resistivity [μΩ·cm] |
Thermal Conductivity [W/(m·K)] |
Coeff. of Linear Thermal Expansion [10-6/K] |
Modulus of Elasticity [GPa] |
Softening Temperature (approx. 10% loss in strength) [°C] |
Melting Temp Range [°C] | |
|---|---|---|---|---|---|---|---|---|---|---|
| [MS/m] | [% IACS] | |||||||||
| CuCr | Cr 0.3 - 1.2 Cu Rest |
8.89 | 26a 48b |
45a 83b |
3.8a 2.1b |
170a 315b |
17 | 112 | ca. 450 | 980 - 1080 |
| CuZr | Zr 0.1 - 0.3 Cu Rest |
8.9 | 35a 52b |
60a 90b |
2.9a 1.9b |
340a | 16 | 135 | ca. 500 | 1020 - 1080 |
| CuCr1Zr CW106C C18150 |
Cr 0.5 - 1.2 Zr 0.03 - 0.3 Cu Rest |
8.92 | 20a 43b |
34a 74b |
5.0a 2.3b |
170a 310 - 330b |
16 | 110a 130b |
ca. 500 | 1070 - 1080 |
Material | Hardness Condi- tion | Tensile Strength Rm [MPa] | 0,2% Yield Strength Rp02 [MPa] | Elongation A50 [%] | Vickers Hardness HV | Spring Bending Limit FFB [MPa] |
|---|---|---|---|---|---|---|
CuCr | R 230a R 400a R 450b R 550b | > 230 > 400 > 450 > 550 | > 80 > 295 > 325 > 440 | 30 10 10 8 | > 55 > 120 > 130 > 150 | 350 |
CuZr | R 260a R 370a R 400b R 420b | > 260 > 370 > 400 > 420 | > 100 > 270 > 280 > 400 | 35 12 12 10 | > 55 > 100 > 105 > 115 | 280 |
CuCr1Zr | R 200a R 400b R 450b | > 200 > 400 > 450 | > 60 > 210 > 360 | 30 12 10 | > 70 > 140 > 155 | 420 |
Copper-Zirconium Alloys
The solubility of Zirconium in copper is 0.15 wt% Zr at the eutectic temperature of 980°C (Figure 10). Copper-zirconium materials have a similar properties spectrum, compared to the one for copper-chromium materials. At room temperature the mechanical properties of copper-zirconium are less suitable than those of copper chromium, its temperature stability is however at least the same.
Copper-Chromium-Zirconium Alloys
The earlier used CuCr and CuZr materials have been partially replaced over the years, by the capitation hardening three materials alloy CuCr1Zr. This material exhibits high mechanical strength at elevated temperatures and good oxidation resistance as well as high softening temperatures. In its hardened condition CuCr1Zr has also a high electrical conductivity (Figure 11). Their usage extends from mechanically and thermally highly stressed parts, such as contact tulips in high voltage switchgear or electrodes for resistance welding.